Abstract
Kidney-derived Madin Darby canine kidney (MDCK) cells form lumina at their apices, and target luminal proteins to an intracellular vacuolar apical compartment (VAC) when prevented from polarizing. Hepatocytes, by contrast, organize their luminal surfaces (the bile canaliculi; BC) between their lateral membranes, and, when nonpolarized, they display an intracellular luminal compartment that is distinct from the VACs of MDCK cells. Overexpression of the serine/threonine kinase Par1b/EMK1/MARK2 induces BC-like lateral lumina and a hepatic-type intracellular luminal compartment in MDCK cells, suggesting a role for Par1b in the branching decision between kidney- and hepatic-type epithelial phenotypes. Here, we report that Par1b promotes lateral lumen polarity in MDCK cells independently of Ca2+-mediated cell–cell adhesion by inhibiting myosin II in a rho kinase-dependent manner. Polarization was inhibited by E-cadherin depletion but promoted by an adhesion-defective E-cadherin mutant. By contrast, apical surface formation in control MDCK cells required Ca2+-dependent cell–cell adhesion, but it occurred in the absence of E-cadherin. We propose that E-cadherin, when in an adhesion-incompetent state at the lateral domain, serves as targeting patch for the establishment of lateral luminal surfaces. E-cadherin depletion also reverted the hepatic-type intracellular luminal compartment in Par1b-MDCK cells to VACs characteristic of control MDCK cells, indicating a novel link between E-cadherin and luminal protein targeting.
INTRODUCTION
A hallmark of nonstratified epithelia is the establishment of luminal domains that are segregated by a belt of occluding junctions from basolateral surfaces. Most epithelia establish the luminal surface at their apices (hence, commonly referred to as apical surfaces). Hepatocyte lumina, the bile canaliculi (BC), by contrast form a connected network of grooves between their lateral surfaces (Fawcett, 1994). In most hepatocytic cell lines capable of polarization, this organization is mimicked by the formation of lateral lumina between two or more cells (Chiu et al., 1990; Ihrke et al., 1993). The mechanisms underlying the formation of distinct columnar or hepatic lumina are unknown.
Studies in cultured kidney (Madin Darby canine kidney; MDCK), mammary (MCF-10A), or intestinal (T84) epithelial cells suggest that lumen formation in columnar cells is intimately linked to a differentiation program initiated by cell–cell contact (Vega-Salas et al., 1987; Vega-Salas et al., 1993; Utech et al., 2005). Cell–cell contact provides spatial cues that serve as landmarks to recruit, in a hierarchical manner, scaffolding and signaling molecules, which define and reinforce luminal, basal, and lateral surface domains by instructing the reorganization of cytoskeletal networks and the establishment of polarized protein-targeting mechanisms (Yeaman et al., 1999; Nelson, 2003). Adhesion molecules of the cadherin family that depend on millimolar concentration of extracellular Ca2+ for their activity have been placed on top of the hierarchy of polarization cues. Thus, when cultured in low (<5 μM) Ca2+ medium, confluent epithelial monolayers not only fail to form cell–cell adhesion junctions but also they lack all aspects of polarization, such as polarized surface domains, occluding junctions, or their epithelial-specific microtubule and actin organization. Raising the Ca2+ concentration to 1.8–2 mM leads to rapid and synchronous development of all aspects of polarity (Vega-Salas et al., 1988; Gonzalez-Mariscal et al., 1990; Mattey et al., 1990). Among the Ca2+-dependent adhesion molecules, E-cadherin has been attributed the principal role as polarization trigger, as function-blocking antibodies to the E-cadherin extracellular domain inhibited the formation not only of adhesion junctions, but also of tight and desmosomal junctions (Gumbiner and Simons, 1987; Gumbiner et al., 1988). Notably, however, even high concentrations of E-cadherin antibodies only delayed but did not prevent the formation of tight junctions in Ca2+-switch assays. More recently, substitution of endogenous E-cadherin for an adhesion-deficient mutant (Troxell et al., 2000, 2001) and Ca2+-switch experiments in E-cadherin–depleted MDCK cells (Capaldo and Macara, 2007) further supported the notion that other Ca2+-dependent adhesion mechanisms can induce the development of tight junctions and polarized surface domains in the absence of E-cadherin–mediated adhesion.
Although MDCK cells establish a bona fide luminal surface only upon cell–cell adhesion, luminal proteins can polarize in the absence of cell–cell contacts. In low Ca2+ medium, they form a specialized intracellular vacuolar apical compartment (VAC), also observed in several intestinal pathologies (Remy et al., 1990; Vega-Salas et al., 1993), that is excreted to give rise to the apical cell surface upon Ca2+ switch (Vega-Salas et al., 1987, 1988). Like MDCK cells in low Ca2+ medium, nonpolarized hepatic WIFB cells accumulate their luminal markers in a specialized intracellular organelle that gives rise to the BC-like luminal domains when polarity is established (Tuma et al., 2002). The hepatic intracellular apical compartment, however, differs from the VACs of MDCK cells in morphology and in the trafficking itineraries for luminal markers: VACs contain microvilli and do not display endocytic markers; they accumulate apical proteins before their appearance at the cell surface (Vega-Salas et al., 1987; Cohen et al., 2004b). The hepatic apical compartment, by contrast, is devoid of actin and is endosomal in nature; luminal markers constitutively cycle between this compartment and the cell surface (Tuma et al., 2002). These differences might reflect the distinct luminal targeting pathways that operate in polarized kidney and hepatic epithelia. Although polarized MDCK cells target most, if not all, of their luminal proteins directly to the apical domain (Powell and Rodriguez-Boulan, 1992; Keller and Simons, 1997; Schuck and Simons, 2006), hepatocytes target many of their BC-markers via transcytosis from the basolateral domain (Hubbard, 1991; Tuma and Hubbard, 2003).
Unlike in MDCK cells, Ca2+-dependent cell–cell contacts alone do not trigger the conversion of the apical endosome into bile-canalicular–like lumina in WIFB cells (Ihrke et al., 1993). The polarization cue for cultured hepatocytes is not known.
We recently reported that the serine/threonine kinase Par1b/MARK2/EMK1, a mammalian orthologue of C. elegans Par1, promotes the formation of BC-like lateral lumina when overexpressed in MDCK cells (Cohen et al., 2004a). Expression of Par1b also caused the appearance of an apical endosomal compartment in nonpolarized cells and a switch from a direct to a transcytotic mode of luminal protein targeting in polarized monolayers, all hallmarks of the liver epithelial phenotype (Cohen et al., 2004b). We suggested that Par1b-MDCK cells represent a model system to elucidate the mechanisms that control the branching between columnar and hepatic polarity pathways. In the current study, we have begun to characterize signaling mechanisms for the Par1b-dependent establishment of hepatic-type polarity in MDCK cells.
MATERIALS AND METHODS
Cell Culture, Transfection, and Virus Transduction
For morphological analysis cells were plated on polycarbonate filters (Transwell; Corning Life Sciences, Acton, MA), or, where indicated, on mouse collagen IV (BD Biosciences, San Jose, CA)-coated coverslips. We observed Par1b-, blebbistatin-, or Y-27632–induced lateral lumen formation also when mechanically dislodged cells were plated on untreated glass coverslips, although with a lower incidence than in filter-grown cells. For biochemical analysis, cells were cultured on plastic tissue culture dishes.
All experiments in which the effect of Par1b was assessed were conducted in a Par1b-MDCK cell line expressing COOH-terminal myc-tagged canine EMK1/Par1b under a doxycycline (dox)-regulated promotor as described previously (Cohen et al., 2004a). The doxycycline-dependent ΔEcadherin-MDCK cell line T151 (Troxell et al., 2000) was provided by Dr. J. Marrs (University of Indiana). For control conditions, expression of the recombinant protein was repressed by 20 ng/ml dox; for Par1b (10-fold over endogenous levels) or ΔEcadherin induction, dox was withdrawn for 24 h before cell seeding. For experiments with the rho-kinase (ROCK) and myosin II inhibitors, we used the parental MDCK-TET-OFF cells (provided by K. Mostov, University of California, San Francisco, San Francisco, CA). For low Ca2+-monolayer cultures and Ca2+-switch experiments, cells were plated at confluence in minimum essential medium modified for suspension (SMEM) (low Ca2+ medium; Invitrogen, Carlsbad, CA) with 10% fetal calf serum dialyzed against phosphate-buffered saline (PBS) and maintained for 16 h before analysis or switch to normal growth medium.
Transduction with recombinant dipeptidyl peptidase IV (DPPIV)-green fluorescent protein (GFP) or Par1b adenovirus was in serum-free SMEM for 2 h with 1 plaque-forming unit (pfu)/cell. Cells were analyzed 12–48 h after adenovirus transduction.
For E-cadherin and α-catenin depletion, Par1b-MDCK cells were transfected with 10 μg of the respective pSUPER-based cDNA by using Amaxa-nucleofection (Amaxa Biosystems, Cologne, Germany) as described previously (Cohen et al., 2004a) and cultured in the presence of dox. Protein depletion was maximal 48–72 h after transfection. For Ca2+-switch experiments, cells were reseeded in SMEM after 36 h and cultured for 16 h in the presence or absence of dox before Ca2+ switch. For experiments in low Ca2+ medium, dox was withdrawn after 36 h in culture, and cells were reseeded in SMEM 24 h later. They were analyzed after 16 h in SMEM.
ΔN90 β-catenin and control vector transfection was via Amaxa-nucleofection into Par1b-MDCK that were cultured for 18 h in the absence of dox before reseeding in SMEM.
cDNA and Adenovirus Generation
Rat DPPIV cDNA (provided by Ora Weisz; Weisz et al., 1992) was cloned into pEGFP-C3 (Clontech, Mountain View, CA). A N-terminal 12-amino acid spacer (KLQSSVSRSRPT) separates the start-Met of DPPIV from the GFP-tag. A recombinant adenovirus expressing the DPPIV-GFP cDNA was generated with the pTrak/pAdenoEasy vector system (He et al., 1998).
Myosin IIA Antisense Treatment
Morpholino antisense oligonucleotides (oligos) were produced by Gene Tools (Philomath, OR). The sequence of the control oligo was (standard control) 5′-CCTCTTACCTCAGTTACAATTTATA-3′; the sequence of the nonmuscle myosin II (myoII)A oligo was 5′-GACTCACAGCCAGGACCTACAGCAG-3′. Cells were transfected with 10 μg of the oligos by Amaxa-nucleofection and cultured for 36 h in DMEM before being reseeded for 12 h at confluence in SMEM. Total cell lysates for immunoblots were prepared 48 h after transfection.
Short Hairpin RNA (shRNA)
The pSUPER-Ecadherin RNA interference (RNAi) construct designed for the target sequence 5′-gtctaacagggacaaagaa-3′ has been validated previously (Qin et al., 2005) and was provided by I. Macara (University of Virginia); the target sequence for the pSUPER-based α-catenin canine RNAi-construct was 5′-cacgaagctgctctccaac-3′. It was designed for the GenBank entry XM_846441.
Antibodies
Antibodies used were as follows: gp135 (clone 3F21D8; provided by G. Ojakian, SUNY Downstate Medical Center, Brooklyn, NY); rat monoclonal zonula occludens (ZO)-1 (Chemicon International, Temecula, CA); E-cadherin monoclonal clone rr1 (Gumbiner and Simons, 1986; provided by the Developmental Studies Hybridoma Bank, Iowa City, IA, as hybridoma); anti-rat DPPIV clone MRC OX-61 (Serotec, Oxford, United Kingdom); Dlg-1, Scribble, ROCKII, Par6, and atypical protein kinase Cζ (aPKCζ) (sc-9961, sc-11049, sc-1851, sc-25525, and sc-216, respectively; Santa Cruz Biotechnology, Santa Cruz, CA); Par3 (Zymed Laboratories, South San Francisco, CA); myosin light chain (MLC)-PS19 and MLC-PT18/19 (Cell Signaling Technology, Danvers, MA); MLC clone MY21 (Sigma-Aldrich, St. Louis, MO); myosin IIA/p200 antibody clone AD7 (Narula et al., 1992); Patj antibody (gift of A. LeBivic, University Marseille, France); α- and β-catenin polyclonal antibodies (Sigma-Aldrich), and hemagglutinin (HA) clone16B12 (Covance, Princeton, NJ).
Immunolabeling Techniques and Immunofluorescence (IF) Analysis
For phospho-MLC (MLC-P) labeling, cells were fixed at 4°C in 3% paraformaldehyde (PFA), permeabilized with 0.2% Triton X-100 (Tx100), and the primary antibody was incubated in PBS with 1% bovine serum albumin (BSA)/10% goat serum overnight at 4°C. For all other IF, MDCK cells were fixed at room temperature with 2% PFA, permeabilized with 0.2% Tx100, and subjected to indirect IF labeling at room temperature. Secondary antibodies were conjugated with Alexa-488, -568, or -633. Rhodamine-labeled phalloidin was used were indicated. Wide-field images were acquired with a 40× dry objective numerical aperture (NA) 0.75 on an upright microscope (model E-600; Nikon, Tokyo, Japan) equipped with a back-illuminated cooled charge-coupled device camera (model 1000-PB; Roper Scientific, Trenton, NJ) and processed with MetaMorph software (Molecular Devices, Sunnyvale, CA). Confocal microscopy was performed with a Leica model TCS SP2 by using a 63× NA 1.4 oil objective. Presented are individual confocal x-y or x-z sections, or where indicated, x-y-z or x-z-y projections generated with LCS software (Leica Microsystems, Deerfield, IL). All image-figures were composed with Adobe Photoshop (Adobe Systems, Mountain View, CA).
Quantification of Lumen Polarity
Quantification of cells exhibiting VACs or lateral lumina in Figures 1, 2, 4, and 6 was from 40× wide-field images of fixed cells that were labeled for gp135, ZO-1, and with rhodamin-phalloidin. Lateral lumina were defined by phalloidin and gp135 accumulation between neighboring cells flanked by a circumferential ring of ZO-1. Graphs are from two experiments with three images (∼400 cells/image) per time point; SEs are presented.
Figure 1.
Par1b promotes lateral lumen polarity in low Ca2+ (SMEM) monolayers. Cells were dislodged with Cellstripper (A–C) or trypsinized and cultured on collagen IV-coated (D) coverslips in SMEM for 18 h. (A) Confocal x-y and x-z sections showing gp135, phalloidin, and ZO-1 in Par1b cells; arrow, lateral lumina surrounded by ZO-1; arrowhead, gp135 in the intracellular apical compartment. (B) DPPIV-GFP (green) and DPPIV ectodomain antibody (red) in unpermeabilized (left) and Tx100-permeabilized (right) control (top) and Par1b (bottom) cells. ZO-1 labeling (blue) occurred after permeabilization. Note that the DPPIV antibody did not have access to intracellular VACs in control cells and intercellular lumina (arrows) in Par1b cells unless they were permeabilized. (C) Merged confocal x-y views of E-cadherin, Dlg, or Scribble and phalloidin- and Z-O1–labeled cells; arrows point to luminal surfaces in Par1b cells; note that the lateral markers are absent from this domain. (D) Confocal images of gp135, phalloidin, and ZO-1 in cells that where trypsinized and cultured on either uncoated or collagen IV (10 μg/cm2)-coated coverslips. Graph, effect of increasing concentration of collagen IV (0–20 μg/cm2) on lateral lumen formation in Par1b cells. Bars, 10 μm.
Figure 2.
ROCK and myosin II inhibition induce lateral lumen polarity in the absence of cell–cell adhesion. MDCK cells dislodged with Cellstripper and cultured at confluence for 16 h in SMEM in the presence of 50 μM blebbistatin, 20 μM Y-27632, or dimethyl sulfoxide (DMSO) 1:2000 (control). Where indicated (bar graph in B), cells were trypsinized and cultured on collagen IV-coated coverslips (10 μg/cm2) for 18 h. (A) Top, confocal x-y and x-z views of gp135, phalloidin, and ZO-1 in control, blebbistatin- and Y-27632–treated cells. Inset, developing lateral lumen between two cells (contours traced in white) labeled for gp135 (green) and ZO-1 (red). Bottom, merged confocal x-y views depicting Scribble, Dlg, and E-cadherin (green) in control and blebbistatin-treated cells. Inset in the scribble panel indicates the absence of scribble from the luminal domain (arrow, gp135 in red). (B) Bar graph, percentage of cells with lateral lumina treated with DMSO, blebbistatin, or Y-27632 or transfected with a control morpholino antisense oligo (contr.MA) or a myosin IIA-morpholino (myoIIA MA). Immunoblots, top, myosin IIA and actin in total lysates from cells expressing control MA (lanes 1 and 3) and myoIIA MA (lanes 2 and 4) representing two different experiments. Bottom, total cell lysates from DMSO-, ML-7–, and Y-27632–treated cells probed for T18/S19 phosphorylation of MLC, S19-MLC phosphorylation and total MLC. Bars, 10 μm.
Figure 4.
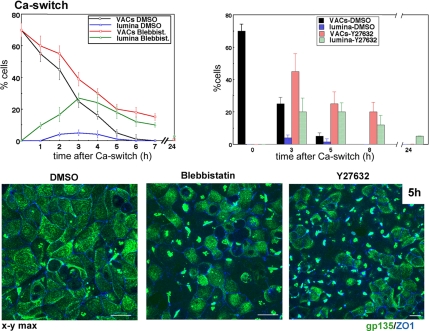
ROCK-mediated myosin II inhibition promotes transient lateral lumina in Ca2+-switch assays. MDCK cells were incubated with DMSO (1:2000), 50 μM blebbistatin, or 20 μM Y-27632 for 30 min in SMEM before and during Ca2+ switch. Merged confocal x-y views of gp135 and ZO-1 5 h after Ca2+ switch. Note the higher incidence of lateral lumina in blebbistatin- and Y-27632–treated monolayers. Graph, percentage of cells with VACs and lateral lumina in DMSO and blebbistatin-treated cells (left) and DMSO- and Y-27632–treated cells (right). Bars, 10 μm.
Figure 6.
Lateral lumen polarity requires the presence of E-cadherin but not E-cadherin–mediated cell–cell adhesion or α-catenin. ΔEcadherin expression was either repressed (+dox) or induced (−dox) in ΔEcad-MDCK cells (black and blue bars in E and a and b in A–C). In B and C, ΔEcad-MDCK cells were transduced with a Par1b-adenovirus. Par1b-MDCK cells were either mock depleted (red bars in E and c in A–C, first panel in D), depleted of E-cadherin (green bars in E and d in A–C, second panel in D) or depleted of α-catenin (e in A–C, third panel in D) and cultured in the presence (A) or absence (B–D) of dox. (A) Merged confocal x-y and x-z views of monolayers 24 h after Ca2+ switch; gp135 (green), ZO-1 (blue), and phalloidin (single x-z section in b, red). Arrowheads indicate the basal surface as determined by phalloidin staining (data not shown). Note that ΔEcad (b) induces lateral lumina at endogenous Par1b levels, whereas Ecadherin depletion (d) allows apical surface formation. α-Catenin–depleted cells (e) fail to elongate a lateral domain, resulting in gp135 accumulation just above the cell base where it localizes along a circumferential actin belt (data not shown; also see Capaldo and Macara, 2007). (B) Merged confocal x-y views and single x-z sections of recombinant Par1b-expressing monolayers 24 h after Ca2+ switch; gp135 (green), ZO-1 (blue), and phalloidin (x-z views, red). Note that E-cadherin depletion (d) but not ΔEcadherin expression (b) inhibits lateral lumen formation. (C) Merged confocal x-y views and single x-z sections of recombinant Par1b-expressing monolayers cultured in low Ca2+ medium in the presence of blebbistatin; gp135 (green), ZO-1 (blue), and phalloidin (x-z view; a, red). Note that E-cadherin depletion (d) but not ΔEcadherin expression (b) or α-catenin depletion (e) leads to apical rather than lateral lumen. (D) Merged confocal x-y views of recombinant Par1b-expressing monolayers 24 h after Ca2+ switch; gp135 (green) and phalloidin (red). Note the presence of gp135- and phalloidin-positive lateral lumina in the control and α-catenin shRNA panels (white arrows), the gp135-positive endosomes in the α-catenin shRNA panel (white asterisk), and gp135- and phalloidin-positive grape-like vacuoles in the E-cadherin shRNA panel (white arrowhead). Graph, percentage of cells with gp135 forming either apical or lateral lumen or in intracellular VACs/endosomes was quantified from three random images of ∼350 cells in each of three independent experiments. SEs are presented. (E) Percentage of cells with lateral lumina 3, 5, and 8 h after Ca2+ switch in the presence of blebbistatin; data from three independent experiments are presented with SE. Asterisk (*) indicates significant difference with p > 0.001. Bars, 10 μm.
Immunoblots
Immunoblots from total cell lysates or kinase assays were probed with primary antibody, rabbit-anti mouse/goat antibodies where appropriate and with 125I-protein A and subjected to PhosphorImager (Typhoon Trio; GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) analysis using IQMac software (GE Healthcare).
ROCK-Kinase Assays
Cells were lysed in 20 mM Tris, pH 8, 150 mM NaCl, 5 mM EDTA, 1% Tx100, 1 mM DTT, 2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 10 μg/ml each of leupeptin, pepstatin, and antipain, 2 mM Na-orthovanadate, 10 mM NaFl, and 25 mM β-glycerophosphate. One milligram of lysate was precleared with 20 μl of Pansorbin (Calbiochem, San Diego, CA) and incubated for 2 h with 2 μg of ROCKII and 4 μg of rabbit anti-goat antibody and an additional 90 min with 10 μl of protein A-Sepharose. Control immunoprecipitations (IPs) were conducted with normal 6 μg of normal rabbit immunoglobulin (IgG). Washed protein A-beads were equilibrated with kinase buffer (10 mM HEPES/KOH, pH 7.0, 150 mM NaCl, 10 mM MgCl2, 1 mM ATP, 0.1% Tx100, 2 mM AEBSF, 10 μg/ml each of leupeptin, pepstatin, and antipain, 2 mM Na-orthovanadate, 25 mM β-glycerophosphate, and 1 mM DTT) and incubated with 30 μl of kinase buffer in the presence of 1.5 μg of recombinant MLC (produced from a recombinant glutathione S-transferase [GST]-fusion protein) for 30 min at 30°C. MLC phosphorylated at S19 (from the supernatants) and ROCKII amounts (from the protein A-beads) was determined by quantitative immunoblots.
Rho-Activity Assays
Rho activity was determined by isolating guanosine triphosphate-bound rho A from cell lysates (prepared from 1 10-cm dish/sample) on immobilized GST-RBD, the GST-tagged rho-binding domain of rhotenkin, exactly as described by Ren et al. (1999). GST-RBD was produced in bacteria and purified on gluthathione-Sepharose by standard procedures. The GST-RBD beads were stored in 10% glycerol at −70°C.
TCF/LEF Reporter Assays
T cell factor (TCF)/lymphoid enhancer binding factor (LEF) reporter assays where performed as described in Elbert et al. (2006). Briefly, cDNAs encoding pRLTK (Renilla luciferase reporter) and either TOPFLASH (firefly luciferase reporter with three LEF-binding domains) or FOPFLASH (LEF-binding defect reporter) cDNAs were cotransfected with shRNA-plasmids. Cells were cultured for 48 h in DMEM, lysed, and assayed for luciferase activities in a luminometer by using the Dual-Luciferase Reporter Assay system (Promega, Madison, WI). The net reporter activity was calculated as the ratio of firefly luciferase reporter activity to Renilla activity. Data are from two experiments with triplicate samples. SEs are indicated.
RESULTS
Par1b Induces MDCK Polarization with Lateral Lumina Independently of Ca2+-mediated Cell–Cell Adhesion in Low Ca2+ Monolayers
Our previous findings suggested that Par1b alters the mechanism of MDCK cell polarization by promoting a pathway that leads to the formation of lateral rather than apical lumina. To identify the step where this branching in polarization occurs, we used our previously described MDCK line that expresses recombinant Par1b under a dox-regulated promotor (Cohen et al., 2004a). These cells allow us to directly compare lumen formation under control conditions (Par1b clone cultured in the presence of dox, referred to as control cells) with that upon Par1b overexpression (Par1b clone cultured in the absence of dox, referred to as Par1b cells).
We focused on contact-naïve cells, i.e., those grown in low Ca2+ medium, because we had previously observed differences in gp135 distribution between control and Par1-MDCK cells even in the absence of cell–cell contacts. Contact-naïve Par1b-MDCK cells accumulated gp135 not in VACs but in endosomes that were in exchange with a substantial cell surface pool of gp135 (Cohen et al., 2004b).
On close inspection, we noticed that ∼10% of Par1b-MDCK cells grown overnight in low Ca2+ medium formed lateral luminal surfaces (Figure 1A, arrow, column 1 in graph). No such polarization events were detected in control cultures (Figure 1, B–D, control). The number of Par1b cells with lateral lumina increased to ∼40% when we seeded cells that were dislodged by nonenzymatic methods (with Cellstripper; Cellgro, Mediatech, Herndon, VA) rather than by trypsinization or when trypsinized cells were plated on collagen IV (Figure 1, graph). Cells with lateral lumina were characterized by the polarized distribution of gp135 and recombinant DPPIV-GFP and labeled heavily for phalloidin, presumably due to the presence of microvilli (Figure 1A, arrow). The luminal surfaces were always flanked by a belt of ZO-1 (Figure 1A). E-cadherin, which is largely removed from the cell cortex in contact-naïve cells (Le et al., 1999; Low et al., 2000), showed increased cortical distribution in Par1b cells. Likewise, the soluble polarity determinants Dlg and Scribble that translocate from the cytoplasm to the lateral cortex upon Ca2+ switch (Reuver and Garner, 1998; Navarro et al., 2005) were enriched at the cortex in contact-naïve Par1b-MDCK cells (Figure 1C). Importantly, all three proteins were excluded from the luminal domains, suggesting that Par1b-MDCK cells had segregated distinct luminal and lateral surface domains in the absence of Ca2+-dependent cell–cell adhesion (Figure 1C, arrows). To determine whether the luminal surfaces were indeed segregated by tight junctions from lateral domains as suggested by ZO-1 labeling, we tested the accessibility of extracellular domain antibodies to the luminal marker DPPIV in DPPIV-GFP–expressing Par1b and control cells. When added to unpermeabilized, fixed cells, DPPIV antibodies had no access to lateral lumina in Par1b cells or to VACs in control cells. Only upon permeabilization with Tx100 did the antibody label the luminal surfaces and VACs that were apparent by GFP fluorescence (Figure 1B). Thus, Par1b cells assemble junctional complexes that prevent the penetration of antibodies from the basolateral to the luminal domain in the absence of Ca2+-mediated cell–cell adhesion. Our data suggest that Par1b, dependent on surface receptor extracellular matrix (ECM) signaling, promotes polarization of MDCK cells independently of Ca2+-dependent cell–cell adhesion.
Inhibition of ROCK and Myosin II Mimics the Cell–Cell Adhesion-independent Polarization Pathway in Par1b-MDCK Cells
Recent data by Eisen et al. (2004) and Ivanov et al. (2005) have implicated ROCK and its target myosin II in apical surface formation in columnar epithelial cells. Lateral lumina formed when ROCK was pharmacologically inhibited during polarization in three-dimensional tubulogenesis assays in MDCK cells and when myosin II was pharmacologically inhibited in Ca2+-switch assays in intestinal derived T84 cells. We, therefore, investigated whether myosin II is linked to the signaling pathway that induces polarization with lateral lumen in contact-naïve MDCK cells.
Addition of 50 μM blebbistatin, a specific inhibitor of myosin II (Straight et al., 2003), to control cells cultured in low Ca2+ medium overnight resulted in 41 ± 5% of cells polarized with lateral lumina that were segregated from the lateral cortical pool of scribble (Figure 2, A and B). As observed for Par1b-MDCK cells, blebbistatin treatment led to an increased recruitment of E-cadherin, Scribble, and Dlg to the cell cortex (Figure 2A). As in Par1b-MDCK cells, the blebbistatin-induced phenotype was dependent on cells being either plated on collagen or dislodged by nonenzymatic methods (Figure 2B).
An antisense-morpholino targeting myosin IIA reduced the levels of this isoform by ∼60% and stimulated lateral lumen formation in low Ca2+ medium cells, albeit to a lesser extent than blebbistatin (Figure 2B). These data suggest that the pharmacological effect is caused by myosin II inhibition; the moderate effect of the morpholino is likely due to the incomplete depletion of myosin IIA and the presence of myosin IIB in MDCK cells.
Myosin II activity depends on the phosphorylation of regulatory MLC on S19 and T18 (Tan et al., 1992). Myosin light chain kinase (MLCK) and the rhoA effector ROCK are chief regulators of phosphorylation of these residues, and they have nonoverlapping functions to activate distinct myosin populations in epithelial (Prahalad et al., 2004) and nonepithelial (Totsukawa et al., 2000) cells. Inhibition of MLCK with 30 μM ML-7 or of ROCK with 20 μM Y-27632 led to a decrease in T18/S19P-MLC in contact-naïve MDCK cells, indicating that both kinases contribute to myosin activity under these conditions (Figure 2B). Yet, only the ROCK inhibitor Y-27632 promoted lateral lumen formation in low Ca2+ monolayers comparable with blebbistatin (38 ± 6%), whereas the MLCK inhibitor ML-7 was without effect (Figure 2, A and B; data for ML-7 not shown). We did not detect distinct subcellular localizations for the enzymes; antibodies to MLCK and ROCKII both gave a diffuse cytoplasmic staining (data not shown). Together, our data indicate that myosin II, controlled by ROCK, allows contacting cells to develop polarity with lateral lumina in the absence of Ca2+-dependent cell–cell adhesion.
Par1b Inhibits MLC-Phosphorylation in Contact-Naïve Cells and Functions Synergistically with Blebbistatin
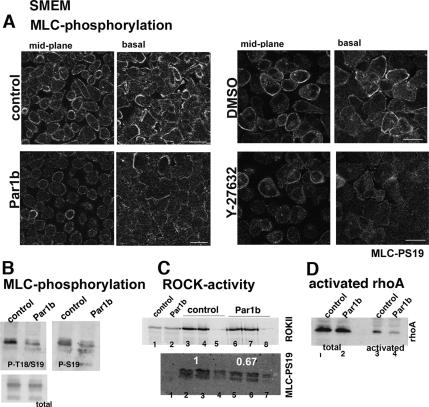
Does Par1b regulate myosin II activity? We determined in quantitative immunoblots that Par1b expression indeed decreased by ∼50% the S19 (52 ± 6% of control; n = 4 experiments) and T18/S19 (45 ± 5% of control; n = 4) phosphorylation of MLC in monolayers grown in low Ca2+ medium (Figure 3B). A decrease in active, S19-phosphorylated MLC was also apparent by immunofluorescence. Par1b-MDCK cells showed reduced MLC-pS19 at the basal lamellipodia and circumferential actin bundles as well as at the cortex of noncontacting surfaces (Figure 3A, see confocal basal and midplanes).
Figure 3.
Par1b inhibits Rho and MLC-phosphorylation in low Ca2+-monolayers. Control and Par1b-MDCK cells (A–D) or DMSO- and Y-27632–treated MDCK cells (A, right) that were cell stripped and cultured in SMEM were analyzed for MLC-phosphorylation (A and B), ROCK activity (C), and activated rho (D). (A) Confocal x-y sections of S19-phosphorylated MLC at the cell center (midplane) and at the basal domain (basal); images were acquired with the same gain and settings; note reduced MLC-P in Par1b cells. (B) Immunoblots of total cell lysates probed for phospho-S19 (P-S19), diphospho-T18+S19 (P-T18/S19), and total MLC (total). (C) ROCK kinase activity as determined from ROCKII-IPs with MLC as substrate. Top, ROCKII immunoblot. Lanes 1 and 2, 50 μg cell lysate; lanes 3 and 4, IP with ROCKII antibody (control); lane 5, IP with normal goat IgG (control); lanes 6 and 7, IP with ROCKII antibody (Par1b); and lane 8, IP with normal goat IgG (Par1b). Bottom, immunoblot of phosphorylated MLC (MLC-PS19). Lane 1, recombinant MLC incubated in reaction buffer alone; lanes 2 and 3, IP with ROCKII antibody (control); lane 4, IP with normal goat IgG (control); lanes 5 and 6, IP with ROCKII antibody (Par1b); and lane 7, IP with normal goat IgG (Par1b). White numbers indicate relative ROCK-activities. The blot is representative of three independent experiments in which the ROCK activity in Par1b cells was 67% (shown), 77 and 63% of that in control cells. (D) Immunoblot depicting total rhoA (lanes 1 and 2) and rhotenkin-GST–bound activated rhoA (lanes 3 and 4). The blot is representative of three experiments decreasing rhotenkin-bound rho in Par1b cells to 45, 51, and 41% of control levels, respectively. Bars, 10 μm.
To determine whether Par1b regulates myosin II activity via the rhoA–ROCK pathway, we isolated active rhoA from cell lysates on immobilized rhotenkin-derived peptide and performed in vitro kinase assays with ROCK immunocomplexes isolated from contact-naïve control and Par1b cells. We measured an averaged 54% decrease in activated rhoA (Figure 3D; 55, 49, and 59% in three independent experiments) and an averaged 31% reduced ROCK activity in Par1b cells (Figure 3C; 33, 23, and 37% in three separate experiments), suggesting that ROCK inhibition at least contributes to the effect of Par1b on myosin II activity. We are currently unable to define whether our in vitro kinase assay underestimates the Par1b-mediated inhibition of ROCK activity and/or whether Par1b regulates myosin activity by additional mechanisms.
To determine whether Par1b and myosin II inhibition function synergistically, we studied the effect of blebbistatin on the polarization of Par1b-MDCK cells in low Ca2+ medium. Although myosin II inhibition and Par1b alone each promoted lumen polarity in ∼40% of the cells, combined they yielded a monolayer with 80 ± 10% (n = 4) polarized cells. Together, these data suggest that Par1b acts in a pathway with rho, ROCK, and myosin II to promote a lateral lumen polarity phenotype in MDCK cells.
Blebbistatin and ROCK Inhibition Promotes Transient Lateral Lumina during MDCK Polarization in Ca2+-Switch Assays
To determine whether myosin II inhibition also mimicked the effect of Par1b on lumen polarity in the presence of Ca2+-dependent cell–cell adhesion, we preincubated control MDCK cells kept in low Ca2+ medium overnight for 30 min before Ca2+ switch with blebbistatin or Y-27632 and maintained the drugs during the polarization process in normal Ca2+ medium. The myosin and ROCK inhibitors delayed exocytosis of VACs, and they noticeably increased the amount of lateral lumina detectable at all time points up to 24 h (18-fold for blebbistatin and 16-fold for Y-27632 at 5 h) (Figure 4). Despite their higher incidence, lateral lumina in myosin II-inhibited cells were transient with a peak occurrence at 4–5 h upon Ca2+ switch, and by 7–8 h, they had mostly given rise to apical surfaces. Thus, the lateral lumen polarity pathway stimulated by myosin II inhibition seems to compete with a cell–cell adhesion-induced apical lumen pathway that eventually supplants it.
The Lateral Lumen Phenotype Requires E-Cadherin Signaling That Is Independent of Cell–Cell Adhesion
The aforementioned findings demonstrate that myosin II inhibition phenocopied Par1b-induced lateral lumen polarity only in the absence but not in the presence of Ca2+-mediated cell–cell adhesion. Thus, we reasoned that in addition to inhibiting myosin II, Par1b antagonizes signaling mechanisms induced by cell–cell adhesion to maintain lateral lumina during Ca2+ switch. To test this assumption, we asked whether inhibition of E-cadherin signaling in conjunction with myosin II inhibition was sufficient to induce the Par1b-phenotype in normal Ca2+ medium.
We used two approaches to interfere with E-cadherin function: 1) shRNA-mediated E-cadherin depletion (Figure 5, A and D) and 2) substitution of endogenous E-cadherin for an adhesion-defective mutant that lacked a large part of its extracellular domain (Troxell et al., 2000, 2001). The recombinant protein, expressed under a doxycycline-regulated promotor, is faithfully targeted to the lateral domain, and it causes increased turnover of its endogenous counterpart (Troxell et al., 2000) (Figure 5, B and D).
Figure 5.
E-cadherin depletion inhibits α-catenin recruitment to the lateral cortex. E-cadherin– (A) and α-catenin (C)–labeled control, E-cadherin- and α-catenin–depleted Par1b cells cultured in the presence of dox. Note that E-cadherin depletion disrupts α-catenin staining at the lateral cortex. (B) E-cadherin (top) and HA (bottom) in ΔEcadherin-MDCK cells cultured in the presence and absence of dox. Note that the E-cadherin antibody (monoclonal rr1) only detects endogenous canine E-cadherin but not the recombinant, HA-tagged truncated E-cadherin derived from the mouse sequence (Troxell et al., 2000). (D) Immunoblots of E-cadherin, α-catenin, and β-catenin in ΔEcad-MDCK cells (+/−dox) and Par1b-MDCK cells transfected with pSUPER (control), shRNA E-cadherin, or shRNA–α-catenin constructs 48 h after dox withdrawal or transfection of shRNA constructs. HA immunoblot detecting the ΔEcadherin construct in ΔEcad-MDCK cells (+/− dox). The asterisk indicates a breakdown product also reported by Troxell et al. (2000). Note that ΔEcadherin and shRNA-E-cadherin expression reduce endogenous E-cadherin levels to similar extents.
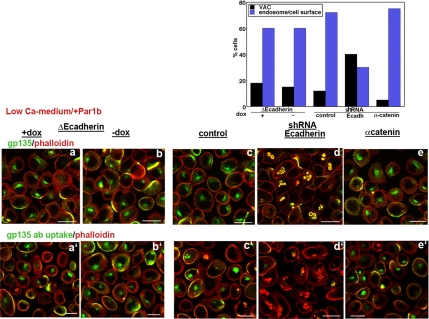
When analyzed in Ca2+-switch experiments, the E-cadherin mutant indeed increased the incidence of lateral lumina in the presence of blebbistatin (Figure 6E, compare black and blue bars) as predicted by our hypothesis. Interestingly, the adhesion mutant caused stable lateral lumen polarity up to 48 h upon Ca2+ switch even in the absence of the myosin inhibitor (Figure 6A, a and b for 24 h; data not shown for 48 h), suggesting that myosin II and E-cadherin function in the same pathway. Unexpectedly, E-cadherin depletion had the opposite effect; it blocked the appearance of even transient lateral lumina in myosin II-inhibited polarizing monolayers (Figure 6E, compare red and green bars). Instead, apical surfaces formed with only slightly delayed kinetics in E-cadherin–depleted control MDCK cells (Supplemental Figure S1). They had a dome-like appearance, as was also observed by Capaldo and Macara (2007), but they showed a polarized apical distribution of both gp135 (Figure 6Ad and Supplemental Figure S1) and DPPIV-GFP (data not shown). Also unexpectedly, E-cadherin depletion, but not expression of the adhesion mutant, led to apical rather than lateral lumen polarity in blebbistatin-treated, low Ca2+ monolayers both in the presence (Figure 6Cd) and absence (data not shown) of recombinant Par1b.
These data indicate that lateral lumen polarity is promoted by the lack of E-cadherin–mediated cell–cell adhesion but that it nevertheless requires adhesion-independent E-cadherin signaling. Apical surfaces, by contrast, can form in the absence of E-cadherin.
The requirement for E-cadherin in lateral lumen formation was furthermore apparent in Par1b-MDCK cells: E-cadherin depletion (but not the E-cadherin mutant) drastically inhibited lateral lumen formation in Ca2+-switch experiments (Figure 6B, compare a and b with c and d). Twenty-four hours after Ca2+ switch, mock-depleted Par1b monolayers were completely polarized (Figure 6Bc and D, control); 62 ± 10% (n = 3) of cells featured lateral lumina, and the rest had apical surfaces. Only 20 ± 5% (n = 3) of E-cadherin–depleted Par1b cells formed lateral lumina, and 44 ± 8% (n = 3) had apical surfaces. Of E-cadherin–depleted Par1b cells, 36 ± 6% (n = 3) accumulated gp135 intracellularly in large, grape-like, F-actin–rich vacuoles (Figure 6Bd and D, E-cadherin shRNA).
E-Cadherin Depletion Alters Apical Protein Trafficking in Nonpolarized Par1b Cells
As discussed in Figure 1, Par1b-MDCK monolayers cultured in the absence of collagen in low Ca2+ medium show few polarized cells; instead, most cells accumulate gp135 in a specialized apical endosome devoid of F-actin (Cohen et al., 2004b; Figures 1A, arrowhead, and 7c; control shRNA in Figure 7, graph). Antibodies to the extracellular domain of gp135 incubated with live cells at 37°C readily labeled the endosomal compartment as it is in equilibration with a cell surface pool (Figure 7c′). Examination of gp135 in E-cadherin–depleted Par1b-MDCK cells before and after Ca2+ switch revealed its localization in fragmented vacuoles that frequently labeled with phalloidin (Figures 7d, shRNA-Ecadherin in Figure 7, graph; and 6D) and were inaccessible to gp135 antibodies (Figure 7d′). These features are hallmarks of VACs characteristic of control MDCK cells.
Figure 7.
E-cadherin depletion alters the targeting of gp135 in nonpolarized Par1b-MDCK cells. ΔEcad-MDCK cells were transduced with Par1b-adenovirus, and ΔEcadherin expression was either repressed (+dox) or induced (−dox) (a, a′, b, b′). Par1b-MDCK cells were either mock depleted (control shRNA, c, c′), depleted of E-cadherin (d, d′), or of α-catenin (e, e′). All monolayers were cultured for 18 h in low Ca2+ medium. a–e, Gp135 total/phalloidin, cells were fixed and labeled for gp135 (green) and with rhodamine-phalloidin. Shown are merged confocal x-y views. Note gp135 and phalloidin labeling only overlay in the E-cadherin shRNA panel. a′–e′, Gp135 ab uptake/phalloidin, gp135 antibodies were incubated with live cells for 1 h at 37°C before fixation and labeling with secondary antibodies (green) and rhodamine-phalloidin. Shown are confocal x-y planes. Note the absence of gp135-antibody (Ab) uptake in the E-cadherin shRNA panel (d′). Graph, percentage of cells with gp135 and F-actin–positive VACs (black bars) or with gp135-positive but F-actin–negative endosomes and gp135 surface staining (blue bars) were determined from 40× confocal images of two independent experiments. In total, ∼300 cells were scored in each experiment. Bars, 10 μm.
As observed for the lumen phenotype, only E-cadherin depletion, but not the adhesion-deficient E-cadherin mutant altered the trafficking itinerary of gp135 in Par1b cells (Figure 7a, a′ and b, b′; ΔEcadherin +/−dox in Figure 7, graph). Thus, adhesion-independent E-cadherin signaling regulates both lumen polarity and apical protein targeting.
E-Cadherin Regulates Lumen Polarity Independently of α- and β-Catenin
To elucidate the E-cadherin signaling mechanisms that regulate the lumen polarity pathway, we evaluated the roles of the E-cadherin–associated proteins α- and β-catenin in lumen formation.
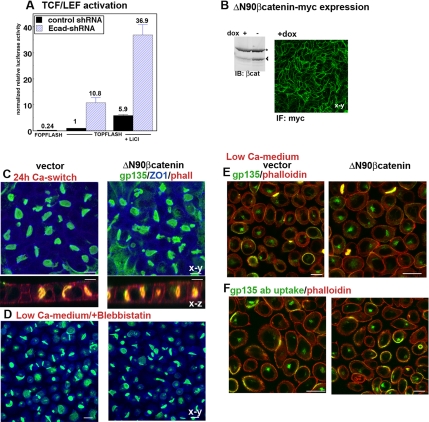
β-Catenin, when not engaged in adhesion complexes, is either degraded or transported to the nucleus where it acts as cotranscription factor with TCF/LEF in the canonical wnt-signaling cascade (Nelson and Nusse, 2004). Using the reporter gene TOPFLASH assay (Molenaar et al., 1996), we determined that E-cadherin depletion indeed drastically increased TCF/LEF-dependent transcription activity (Figure 8A). This increase was apparent both in the presence and absence of LiCl, an inhibitor of glycogen synthase kinase 3β (GSK3β) that stimulates wnt signaling by preventing GSK3β-mediated β-catenin degradation. To assess whether stimulation of wnt signaling interferes with lateral lumen polarity, we expressed in Par1b-MDCK cells a stabilized form of β-catenin that is resistant to GSK3β-induced degradation (Figure 8B). We have previously demonstrated that this recombinant protein stimulates TCF/LEF transcription activity in MDCK cells in TOPFLASH assays (Elbert et al., 2006). Consistent with our findings from this earlier study, we detected no effect of βcatenin-ΔN90 on lateral lumen formation in Ca2+-switch assays (Figure 8C). Likewise, there was no inhibition of lateral lumen formation in the absence of Ca2+-mediated cell–cell adhesion (Figure 8D) and most nonpolarized Δ90Nβcatenin-cells maintained gp135 in F-actin–negative endosomes rather than VACs (Figure 8, E and F). Thus, E-cadherin depletion does not seem to inhibit lateral lumina in Par1b cells by stimulating canonical wnt signaling.
Figure 8.
ΔN90β-catenin expression does not alter lumen formation or gp135 trafficking in Par1b cells. (A) TOPFLASH or FOPFLASH control assays of MDCK cells cotransfected with either pSUPER-control plasmid or the E-cadherin shRNA-plasmid and cultured for 48 h. Where indicated, cells were treated with 20 mM LiCl 12 h before cells lysis. Normalized luciferase activity is expressed as–fold increase over the baseline activity in vector-transfected cells. (B–F) Par1b cells were transfected either with control vector cDNA or cDNA encoding myc-tagged ΔN90-β-catenin and cultured in the presence (B and C) or absence (D–F) of dox. All monolayers were cultured for 18 h in low Ca2+ medium. (B) β-Catenin-immunoblot of Par1b cells cultured either in the presence or absence of dox (+/−dox) depicting recombinant myc- ΔN90β-catenin (arrowhead) and endogenous β-catenin (asterisk). Myc-ΔN90 β-catenin staining of Par1b cells in +dox; myc-ΔN90 β-catenin distribution could not be analyzed in Par cells cultured in −dox due to the presence of myc-Par1b, but the immunoblot indicates comparable ratios of wild type to stabilized β-catenin in −dox and +dox cells. (C) merged confocal x-y and single x-z views of monolayers 24 h after Ca2+ switch; gp135 (green), ZO-1 (blue), and phalloidin (x-z views, red). Note undiminished lateral lumen formation in myc-ΔN90 β-catenin cells. (D) Merged confocal x-y views of monolayers cultured in low Ca2+ medium in the presence of blebbistatin; gp135 (green), and ZO-1 (blue). Note lateral lumina in both control and myc-ΔN90 β-catenin cells. (E and F) Cells were cultured in low Ca2+ medium for 18 h, and total gp135/phalloidin staining (E) and gp135 antibody uptake (F) were analyzed as described in Figure 7. Bars, 10 μm.
α-Catenin associates with actin and actin-binding proteins and participates in the microfilament reorganization induced by E-cadherin–mediated cell–cell adhesion (Bershadsky, 2004; Scott and Yap, 2006; Weis and Nelson, 2006). When analyzed 24 h after Ca2+ switch, E-cadherin–depleted cells featured less α-catenin at the lateral cortex than control cells as judged by immunofluorescence (Figure 5C).
shRNA-mediated reduction of α-catenin levels in Par1b-MDCK cells (Figure 5, C and D) significantly delayed the establishment of luminal surfaces. Twenty-four hours after Ca2+ switch, 52 ± 9% of the cells still maintained gp135 intracellularly, but unlike in E-cadherin–depleted cells, lumina always formed between contacting cells and never at the apex (Figure 6Bd and D). Also unlike E-cadherin depletion, α-catenin shRNA did not induce apical lumina in low Ca2+ medium. Similar to control monolayers, α-catenin–depleted Par1b cells formed lateral lumina in low Ca2+ medium when treated with blebbistatin (Figure 6Ce). α-Catenin depletion also did not alter the trafficking itinerary of gp135 in nonpolarized Par1b cells in low Ca2+ medium as determined by gp135-antibody uptake and the absence of phalloidin staining of the gp135-positive endosomes (Figure 7e, e′, shRNA α-catenin; and graph). This phenomenon was also apparent for the population of nonpolarized cells 24 h after Ca2+ switch: intracellular gp135 in α-catenin–depleted cells was present mostly in F-actin–negative endosomes in contrast to the F-actin–positive vacuoles characteristic of nonpolarized cells lacking E-cadherin (Figure 6D). Thus, although α-catenin and E-cadherin depletion both inhibit the establishment of a luminal cell surface domain in Par1b-MDCK cells, lack of cortical α-catenin does not account for the change in lumen position and gp135 trafficking caused by E-cadherin depletion.
DISCUSSION
We report that Par1b promotes the polarization of MDCK cells independently of Ca2+-mediated cell–cell adhesion, which is an obligate trigger of polarization in control cells. As in normal Ca2+ medium, Par1b MDCK cells maintained in low Ca2+ medium established lateral luminal surfaces reminiscent of the hepatic polarity phenotype. Luminal and lateral markers were always segregated by a junctional belt composed of tight junction markers such as ZO-1 and occludin (data not shown) as well as associated apical junctional proteins such as Par3, Par6, aPKC, and Patj (data not shown). Unlike the kinase LKB1/Par4 that has been reported to promote epithelial polarity in a cell autonomous manner (Baas et al., 2004), Par1b-induced polarization required cell–cell contacts and did not occur in single cells (data not shown).
We identified myosin II as a Par1b target responsible for the cell–cell adhesion independent polarization process. Inhibition of myosin II or of the myosin activator rho-kinase mimicked the Par1b phenotype, and Par1b inhibited the activating phosphorylation on Ser19/Thr18 of myosin light chain by 50%. Consistent with a rho-dependent mechanism of myosin II inhibition, we detected 50% less active rho in contact-naïve Par1b monolayers and a reproducible, although smaller, decrease in rho-kinase activity.
Myosin inhibition in low Ca2+ monolayers relaxes tension at the cortex, where MLC is active in control cells (Castillo et al., 1998; also see Figure 3A) and could help adjacent cells to make intimate cell–cell contacts necessary for the formation of apical junctional complexes, a likely prerequisite for the establishment of distinct surface domains. Myosin inhibition most likely has additional effects that promote the targeting of luminal markers and/or preassembled luminal domains such as VACs to cell–cell contact sites. This is suggested by our observation that the induction of apical junctional complexes is not by itself sufficient to establish polarized surface domains. Phorbol ester-induced PKC activation in MDCK monolayers promoted tight junction formation in low Ca2+ medium (Balda et al., 1993), but it did not induce polarized luminal domains (Cohen and Müsch, unpublished observations). Myosin II-dependent events are likely linked to cell–ECM signaling processes. Par1b and myosin II inhibitors stimulate lumen formation only when trypsin-sensitive cell surface receptors remain intact. Those receptors might be involved in ECM signaling, because lumen formation in trypsinized cells can be rescued by collagen IV. Myosin activity is known to serve as regulator of focal adhesion signaling (Smilenov et al., 1999; Fincham et al., 2000; Delanoe-Ayari et al., 2004) and is itself regulated by cell–substrate interactions (Fincham et al., 2000; Polte et al., 2004; de Rooij et al., 2005).
Although independent of Ca2+-mediated cell–cell adhesion, the Par1b-induced lateral lumen polarity phenotype nevertheless required the presence of E-cadherin. How could E-cadherin regulate lumen polarity? On Ca2+ withdrawal or in noncontacting epithelial cells, E-cadherin is largely removed from the cell surface, and it is associated with intracellular organelles (Le et al., 1999). During cell contact formation in the presence of Ca2+, the protein is first delivered to the plasma membrane where it progressively concentrates at sites of cell–cell contact as adhesion junctions assemble and mature (Adams et al., 1998). Lateral dimerization and concentration of E-cadherin into larger-scale clusters via interactions of their ectodomains in cis is crucial for homophilic cell-cell adhesion in trans (Brieher et al., 1996; Yap et al., 1997). The E-cadherin clustering is also regulated by signaling events at the cytoplasmic face (Yap et al., 1998). Thus, once engaged in homophilic interactions, E-cadherin recruits myosin II to the lateral cortex, which in turn promotes lateral E-cadherin clustering and further strengthens cell–cell adhesion (Shewan et al., 2005). Myosin II inhibition or substitution of endogenous E-cadherin for a mutant deficient in homophilic ligation and lateral clustering promoted lateral lumina in Ca2+-switch assays. In addition, we observed that myosin II inhibitors and Par1b increased the population of (adhesion-incompetent) E-cadherin at sites of cell–cell contact in low Ca2+ medium. Based on these findings, we propose that the presence of E-cadherin at cell–cell contact sites in an adhesion-incompetent state serves as targeting patch for the establishment of lateral luminal surfaces flanked by a junctional belt perpendicular to the basal domain (see model in Figure 9).
Figure 9.
Model for the role of Par1b, myosin II, and E-cadherin in lumen formation. Low Ca2+ medium. 1) In low Ca2+ medium, control MDCK cells remain nonpolarized: E-cadherin (ochre) accumulates in intracellular endosomes and luminal markers (green) are retained in VACs. 2) Recombinant Par1b promotes polarity with lateral lumina under these conditions by inhibiting myosin II in a rhoA- and ROCK-dependent manner. Myosin inhibition causes E-cadherin recruitment to cell–cell contact sites where the latter remains adhesion incompetent and serves as targeting patch for luminal proteins and the formation of a vertical belt of apical junctional complexes (blue). E-cadherin is absent from established luminal domains. Ca2+-dependent cell–cell contacts. 3) Addition of Ca2+ to control monolayers stimulates the recruitment of E-cadherin to the cell cortex and a positive feedback loop between E-cadherin and myosin II that promotes E-cadherin clustering in cis and adhesion in trans (Shewan et al., 2005). 3 and 4) Apical lumen formation accompanies the establishment of E-cadherin–based junctions, but it can occur in the absence of E-cadherin, presumably promoted by other Ca2+-dependent signaling events. 5) By inhibiting myosin II in polarizing cells, Par1b impedes lateral clustering of E-cadherin and adhesion junction formation. The resulting cortical adhesion incompetent E-cadherin molecules serve as cue for the establishment of lateral luminal domains as observed in Par1b cells cultured in low Ca2+ medium. Adhesion junctions form at cell–cell contacting sites adjacent to the lumen.
Inhibition of E-cadherin adhesive function, however, does not seem to be sufficient for maintaining stable lateral lumen polarity. Myosin II inhibition promoted lateral lumina only transiently 3–5 h after Ca2+ switch (Figure 4), and even long-term cultures of ΔE-cadherin cells in 3D-collagen matrices resulted in cysts with apical lumina (Troxell et al., 2001). It is conceivable, therefore, that Par1b promotes the establishment of lateral lumina in polarizing cells by inhibiting myosin II but maintains this polarity phenotype through additional signaling mechanisms. These might involve the re-enforcement of adhesion junctions adjacent to the lumina, as we had observed previously that Par1b promoted cell–cell adhesion in hanging drop-assays (Elbert et al., 2006). Alternatively, Par1b could inhibit actin remodeling processes that arrange the circumferential actin belt at the cell apex. Notably, depletion of α-catenin, an adhesion molecule thought to play a key role in actin organization at the lateral domain, decreased from 36 to 0 the percentage of Par1b cells displaying apical luminal surfaces (Figure 6D), hinting at an involvement of microfilaments in lumen positioning.
Depletion of E-cadherin had strikingly different effects on lumen formation in Par1b and control monolayers in Ca2+-switch experiments. Apical surfaces in control MDCK cells formed with only slightly delayed kinetics, a conclusion also reached by (Capaldo and Macara, 2007). These authors determined that lack of E-cadherin did not induce or alter the expression of other cadherins in MDCK cells, suggesting that K-cadherin and/or the desmosomal cadherins expressed in MDCK cells are sufficient to mediate the Ca2+-dependent polarization process, although with delayed kinetics. Lumen formation in Par1b cells, by contrast, was severely compromised and gp135 accumulated in large, grapelike vacuoles (Figure 6D) that we observed even 48 h after Ca2+ switch and in E-cadherin–depleted Par1b monolayers that were not preincubated in low Ca2+ medium (data not shown).
VACs were also apparent when E-cadherin–depleted Par1b cells were cultured in low Ca2+ medium. This is in contrast to Par1b control cells that maintain gp135 in specialized apical endosomes also found in nonpolarized hepatocytes, Thus, in addition to inhibiting lumen formation, E-cadherin depletion also reversed the change in apical protein trafficking induced by Par1b overexpression in MDCK cells. The E-cadherin signaling mechanisms responsible for both phenotypes remain to be elucidated. We have focused here on α- and β-catenin, components of the “core adhesion complex” that showed altered subcellular distribution in response to E-cadherin depletion. However, neither a stabilized β-catenin mutant that mimicked the increase in nuclear β-catenin nor RNAi-mediated depletion of α-catenin phenocopied E-cadherin depletion. The elucidation of the molecular basis for this novel link between E-cadherin and apical protein trafficking will, therefore, be subject of future work.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. E. Rodriguez-Boulan (Cornell University Medical College, NY) for support. This study was funded in part by National Institutes of Health grant GM-34107 to E. Rodriguez-Boulan. We thank Dr. A. LeBivic (University of Marseille, France) for the Patj antibody, Dr. A. Rajasekaran (University of California-Los Angeles) for providing the GST-RBD cDNA, Dr. O. Weisz (University of Pittsburgh) for the cDNA encoding rat DPPIV, Dr. I. Macara (University of Virginia, Charlottesville, VA) for the pSUPER-Ecadherin plasmid, and Dr. J. Marrs (Indiana University, Indianapolis, IN) for providing the ΔE-cadherin-cell line (T151). We thank Dr. A. Reilein (Cornell University Medical College, NY) for helpful comments on the manuscript. The hybridoma rr1 developed by Dr. B. Gumbiner (University of Virginia, Charlottesville, VA) were obtained from the Developmental Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa. This work was supported by grant SDG 0235130N from the American Heart Association and National Institutes of Health grant R01 DK064842 (to A.M.) and by funds from the M. Dyson Foundation.
Abbreviations used:
- BC
bile canaliculi
- dox
doxycycline
- DPPIV
dipeptidyl aminopeptidase IV
- ECM
extracellular matrix
- IF
immunofluorescence
- IP
immunoprecipitation
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MyoII
nonmuscle myosin II
- ROCK
rho-kinase
- SMEM
minimum essential medium modified for suspension
- VAC
vacuolar apical compartment.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0095) on April 4, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Adams C. L., Chen Y. T., Smith S. J., Nelson W. J. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas A. F., Kuipers J., van der Wel N. N., Batlle E., Koerten H. K., Peters P. J., Clevers H. C. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- Balda M. S., Gonzalez-Mariscal L., Matter K., Cereijido M., Anderson J. M. Assembly of the tight junction: the role of diacylglycerol. J. Cell Biol. 1993;123:293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A. Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Brieher W. M., Yap A. S., Gumbiner B. M. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C. T., Macara I. G. Depletion of e-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo A. M., Lagunes R., Urban M., Frixione E., Meza I. Myosin II-actin interaction in MDCK cells: role in cell shape changes in response to Ca2+ variations. J. Muscle Res. Cell Motil. 1998;19:557–574. doi: 10.1023/a:1005316711538. [DOI] [PubMed] [Google Scholar]
- Chiu J. H., Hu C. P., Lui W. Y., Lo S. C., Chang C. M. The formation of bile canaliculi in human hepatoma cell lines. Hepatology. 1990;11:834–842. doi: 10.1002/hep.1840110519. [DOI] [PubMed] [Google Scholar]
- Cohen D., Brennwald P. J., Rodriguez-Boulan E., Musch A. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J. Cell Biol. 2004a;164:717–727. doi: 10.1083/jcb.200308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D., Rodriguez-Boulan E., Musch A. Par-1 promotes a hepatic mode of apical protein trafficking in MDCK cells. Proc. Natl. Acad. Sci. USA. 2004b;101:13792–13797. doi: 10.1073/pnas.0403684101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J., Kerstens A., Danuser G., Schwartz M. A., Waterman-Storer C. M. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J. Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanoe-Ayari H., Al Kurdi R., Vallade M., Gulino-Debrac D., Riveline D. Membrane and acto-myosin tension promote clustering of adhesion proteins. Proc. Natl. Acad. Sci. USA. 2004;101:2229–2234. doi: 10.1073/pnas.0304297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen R., Ratcliffe D. R., Ojakian G. K. Modulation of epithelial tubule formation by Rho kinase. Am. J. Physiol. 2004;286:C857–C866. doi: 10.1152/ajpcell.00246.2003. [DOI] [PubMed] [Google Scholar]
- Elbert M., Cohen D., Musch A. PAR1b promotes cell-cell adhesion and inhibits Dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol. Biol. Cell. 2006;17:3345–3355. doi: 10.1091/mbc.E06-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. A Textbook of Histology. In: Bloom W., Fawcett D. W., editors. London, United Kingdom: Chapman and Hall; 1994. pp. 57–81. [Google Scholar]
- Fincham V. J., James M., Frame M. C., Winder S. J. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Contreras R. G., Bolivar J. J., Ponce A., Chavez De Ramirez B., Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am. J. Physiol. 1990;259:C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J. Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. The role of uvomorulin in the formation of epithelial occluding junctions. Ciba Found. Symp. 1987;125:168–186. doi: 10.1002/9780470513408.ch11. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T. C., Zhou S., da Costa L. T., Yu J., Kinzler K. W., Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. L. Targeting of membrane and secretory proteins to the apical domain in epithelial cells. Semin. Cell Biol. 1991;2:365–374. [PubMed] [Google Scholar]
- Ihrke G., Neufeld E. B., Meads T., Shanks M. R., Cassio D., Laurent M., Schroer T. A., Pagano R. E., Hubbard A. L. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J. Cell Biol. 1993;123:1761–1775. doi: 10.1083/jcb.123.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., Hunt D., Utech M., Nusrat A., Parkos C. A. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P., Simons K. Post-Golgi biosynthetic trafficking. J. Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Le T. L., Yap A. S., Stow J. L. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Miura M., Roche P. A., Valdez A. C., Mostov K. E., Weimbs T. Intracellular redirection of plasma membrane trafficking after loss of epithelial cell polarity. Mol. Biol. Cell. 2000;11:3045–3060. doi: 10.1091/mbc.11.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattey D. L., Burdge G., Garrod D. R. Development of desmosomal adhesion between MDCK cells following calcium switching. J. Cell Sci. 1990;97:689–704. doi: 10.1242/jcs.97.4.689. [DOI] [PubMed] [Google Scholar]
- Molenaar M., van de Wetering M., Oosterwegel M., Peterson-Maduro J., Godsave S., Korinek V., Roose J., Destree O., Clevers H. XTcf-3 transcription factor mediates beta catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Narula N., McMorrow I., Plopper G., Doherty J., Matlin K. S., Burke B., Stow J. L. Identification of a 200-kD, brefeldin-sensitive protein on Golgi membranes. J. Cell Biol. 1992;117:27–38. doi: 10.1083/jcb.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C., et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- Nelson W. J. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polte T. R., Eichler G. S., Wang N., Ingber D. E. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am. J. Physiol. 2004;286:C518–C528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- Powell S., Rodriguez-Boulan E. Epithelial Organization and Development. In: Fleming T. P., editor. London, United Kingdom: Chapman & Hall; 1992. pp. 89–110. [Google Scholar]
- Prahalad P., Calvo I., Waechter H., Matthews J. B., Zuk A., Matlin K. S. Regulation of MDCK cell-substratum adhesion by RhoA and myosin light chain kinase after ATP depletion. Am. J. Physiol. 2004;286:C693–C707. doi: 10.1152/ajpcell.00124.2003. [DOI] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B. M., Macara I. G. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy L., Gorvel J. P., Jacquier M. F., Rigal A., Davoust J. Confocal microscopy as a tool to reveal the tridimensional organization of intracellular lumens and intercellular cysts in a human colon adenocarcinoma cell line. Biol. Cell. 1990;69:129–138. doi: 10.1016/0248-4900(90)90339-5. [DOI] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B., Schwartz M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuver S. M., Garner C. C. E-cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J. Cell Sci. 1998;111:1071–1080. doi: 10.1242/jcs.111.8.1071. [DOI] [PubMed] [Google Scholar]
- Schuck S., Simons K. Controversy fuels trafficking of GPI-anchored proteins. J. Cell Biol. 2006;172:963–965. doi: 10.1083/jcb.200603015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. A., Yap A. S. Cinderella no longer: α-catenin steps out of cadherin's shadow. J. Cell Sci. 2006;119:4599–4605. doi: 10.1242/jcs.03267. [DOI] [PubMed] [Google Scholar]
- Shewan A. M., Maddugoda M., Kraemer A., Stehbens S. J., Verma S., Kovacs E. M., Yap A. S. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilenov L. B., Mikhailov A., Pelham R. J., Marcantonio E. E., Gundersen G. G. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- Straight A. F., Cheung A., Limouze J., Chen I., Westwood N. J., Sellers J. R., Mitchison T. J. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Tan J. L., Ravid S., Spudich J. A. Control of nonmuscle myosins by phosphorylation. Annu. Rev. Biochem. 1992;61:721–759. doi: 10.1146/annurev.bi.61.070192.003445. [DOI] [PubMed] [Google Scholar]
- Totsukawa G., Yamakita Y., Yamashiro S., Hartshorne D. J., Sasaki Y., Matsumura F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell M. L., Gopalakrishnan S., McCormack J., Poteat B. A., Pennington J., Garringer S. M., Schneeberger E. E., Nelson W. J., Marrs J. A. Inhibiting cadherin function by dominant mutant E-cadherin expression increases the extent of tight junction assembly. J. Cell Sci. 2000;113:985–996. doi: 10.1242/jcs.113.6.985. [DOI] [PubMed] [Google Scholar]
- Troxell M. L., Loftus D. J., Nelson W. J., Marrs J. A. Mutant cadherin affects epithelial morphogenesis and invasion, but not transformation. J. Cell Sci. 2001;114:1237–1246. doi: 10.1242/jcs.114.6.1237. [DOI] [PubMed] [Google Scholar]
- Tuma P. L., Hubbard A. L. Transcytosis: crossing cellular barriers. Physiol. Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- Tuma P. L., Nyasae L. K., Hubbard A. L. Nonpolarized cells selectively sort apical proteins from cell surface to a novel compartment, but lack apical retention mechanisms. Mol. Biol. Cell. 2002;13:3400–3415. doi: 10.1091/mbc.02-04-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utech M., Ivanov A. I., Samarin S. N., Bruewer M., Turner J. R., Mrsny R. J., Parkos C. A., Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Rodriguez-Boulan E. Modulation of the expression of an apical plasma membrane protein of Madin-Darby canine kidney epithelial cells: cell-cell interactions control the appearance of a novel intracellular storage compartment. J. Cell Biol. 1987;104:1249–1259. doi: 10.1083/jcb.104.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): a cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J. Cell Biol. 1988;107:1717–1728. doi: 10.1083/jcb.107.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., San Martino J. A., Salas P. J., Baldi A. Vacuolar apical compartment (VAC) in breast carcinoma cell lines (MCF-7 and T47D): failure of the cell-cell regulated exocytosis mechanism of apical membrane. Differentiation. 1993;54:131–141. doi: 10.1111/j.1432-0436.1993.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Nelson W. J. Re-solving the cadherin-catenin-actin conundrum. J. Biol. Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz O. A., Machamer C. E., Hubbard A. L. Rat liver dipeptidylpeptidase IV contains competing apical and basolateral targeting information. J. Biol. Chem. 1992;267:22282–22288. [PubMed] [Google Scholar]
- Yap A. S., Brieher W. M., Pruschy M., Gumbiner B. M. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr. Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Yap A. S., Niessen C. M., Gumbiner B. M. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J. Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K., Nelson W. J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.