Abstract
Using a mutant hepatocyte cell line in which E-cadherin and β-catenin are completely depleted from the cell surface, and, consequently, fail to form adherens junctions, we have investigated adherens junction requirement for apical–basolateral polarity development and polarized membrane trafficking. It is shown that these hepatocytes retain the capacity to form functional tight junctions, develop full apical–basolateral cell polarity, and assemble a subapical cortical F-actin network, although with a noted delay and a defect in subsequent apical lumen remodeling. Interestingly, whereas hepatocytes typically target the plasma membrane protein dipeptidyl peptidase IV first to the basolateral surface, followed by its transcytosis to the apical domain, hepatocytes lacking E-cadherin–based adherens junctions target dipeptidyl peptidase IV directly to the apical surface. Basolateral surface-directed transport of other proteins or lipids tested was not visibly affected in hepatocytes lacking E-cadherin–based adherens junctions. Together, our data show that E-cadherin/β-catenin–based adherens junctions are dispensable for tight junction formation and apical lumen biogenesis but not for apical lumen remodeling. In addition, we suggest a possible requirement for E-cadherin/β-catenin–based adherens junctions with regard to the indirect apical trafficking of specific proteins in hepatocytes.
INTRODUCTION
Epithelial cells are characterized by their asymmetric cell surface organization, which includes an apical domain that faces the lumen, a basolateral domain facing the underlying tissue, and a lateral domain facing neighboring cells. Cell surface asymmetry, coupled to the polarized distribution of intracellular organelles and cytoskeleton, as well as to cellular processes such as proliferation, is crucial for epithelial functioning. Perturbed or loss of epithelial asymmetry is a hallmark of many epithelial diseases, including carcinogenesis. The process by which epithelial cells develop apical-basolateral surface asymmetry is still poorly understood (Le Bivic et al., 2005). Nectin- and E-cadherin–mediated cell-cell adhesion is thought to serve as an initial cue for the development of apical–basolateral cell polarity by first defining the lateral plasma membrane. Studies in cultured epithelial cells (Vega-Salas et al., 1987, McNeill et al., 1990; Miyoshi and Takai, 2005) and Drosophila (Tepass and Hartenstein, 1994) suggest that E-cadherin induces the formation of a primordial junction complex that subsequently matures (i.e., forms a multiprotein complex) and eventually evolves into distinct and spatially separated adherens junctions (AJs) and tight junctions (TJs; Miyoshi and Takai, 2005). TJs then allow for the spatial segregation of apical and basolateral surface-enriched proteins and lipids within the plasma membrane bilayer by acting as a physical barrier, and the separation of the extracellular apical and basolateral milieus by acting as a diffusion barrier (van Meer and Simons 1986). It is thought that the formation of TJs generally requires prior formation and maintenance of AJs (Miyoshi and Takai, 2005, and references herein).
In addition to a role for E-cadherin in the assembly of AJs and TJs, E-cadherin–mediated cell–cell adhesion and the subsequent remodeling of the actin and microtubule cytoskeleton have been proposed to give rise to basolateral but not apical targeting patches for intracellular trafficking pathways originating from the Golgi apparatus (Yeaman et al., 1999; 2004). This is exemplified by autosomal dominant polycystic kidney disease, characterized by perturbations in the polarized phenotype and function of epithelial cells, in which mutated polycystin proposedly disrupts E-cadherin–dependent cytoarchitecture, and, in this way, adversely affect protein assemblies that are crucial for basolateral but not apical trafficking (Charron et al., 2000).
In this study, we have investigated AJ requirement for apical–basolateral polarity development and polarized membrane trafficking, by taking advantage of a mutant hepatocyte cell line, HepG2-AJ−, in which E-cadherin and β-catenin are completely depleted from the cell surface; consequently, they fail to form AJs. Specifically, HepG2-AJ− cells express a mutant form of the cell cycle regulatory protein p27(Kip1) that cannot be phosphorylated at its serine-10. In these cells, β-catenin interacts with p27 and is prevented from interacting with E-cadherin (Théard, Raspe, Kalicharan, Hoekstra, and van IJzendoorn, unpublished data). This coincides with the intracellular retention of E-cadherin, in accordance with the proposed role of β-catenin acting as a chauffeur to facilitate the transport of E-cadherin out of the endoplasmic reticulum to the plasma membrane (Chen et al., 1999). By using these cells, it is shown that E-cadherin-mediated cell–cell adhesion is dispensable for TJ formation and apical–basolateral polarity development. Subsequent apical lumen remodeling, however, is inhibited in these cells. In addition, it is shown that in these cells the initial basolateral targeting of specific apical proteins is inhibited and that these proteins are targeted directly to the apical surface. The implications of these findings with regard to our understanding of cell polarity and polarized membrane trafficking are discussed.
MATERIALS AND METHODS
Cell Culture
HepG2 cells (ATCC HB8065) were cultured in DMEM with 4500 mg/l glucose and supplemented with 10% fetal calf serum and antibiotics. Culture media were changed every other day. The creation of the clonal HepG2-AJ− cell line will be described in another study. HepG2-AJ− cells were cultured in medium supplemented with 1 mg/ml G-418 (Geneticin; Invitrogen, Carlsbad, CA).
Fluorescence Microscopy
Cells cultured on glass coverslips for 3 d were fixed and stained as described in van der Wouden et al. (2002). The antibodies used were mouse monoclonal anti-β-catenin (BD Transduction Laboratories, Lexington, KY), rabbit polyclonal anti-E-cadherin (kindly provided by M. Wheelock, Eppley Cancer Center, University of Nebraska Medical Center, Omaha, NE), mouse monoclonal anti-multidrug resistance protein 1 (MDR1) (C219; Abcam, Cambridge, MA), polyclonal anti-radixin (Sigma-Aldrich, St. Louis, MO), mouse monoclonal anti-5′nucleotidase, mouse monoclonal anti-dipeptidylpeptidase (DPP)IV (gift from Dr. Hauri, Biozentrum der Universität Basel, Basel, Switzerland), mouse monoclonal anti-zonula occludens (ZO)-1 (Zymed Laboratories, South San Francisco, CA), rabbit polyclonal anti-protease–activated receptor-3 (PAR3) (Upstate Biotechnology, Lake Placid, NY), and Alexa-Fluor 488 or -596 goat anti-rabbit or anti-mouse (Invitrogen, Carlsbad, CA) as secondary antibodies. Hoechst 33528 (5 ng/ml) was used to stain the nuclei. In the E-cadherin blocking experiments, cells were cultured in presence of E-cadherin blocking antibodies (1:50; gift from M. Wheelock) for the indicated times, fixed, and processed for microscopy.
For cell polarity determination, the cells were fixed with acetone at −20°C for 5 min and immunostained with monoclonal anti-villin antibodies. The degree of cell polarity was determined by counting the number of apical structures (which can be either intracellular vacuolar apical compartments [VACs], or intercellular bile canalicular lumens [BCs]; see text) per 100 nuclei. F-actin staining of apical structures was done as described in van der Wouden et al. (2002). Cells were examined with an Olympus Provis AX70 fluorescence microscope.
Electron Microscopy
Cells were washed with 6.8% saccharose to remove serum in 0.1 M cacodylate buffer, pH 7.4, at room temperature (RT) and fixed for 30 min at RT with 2% glutaraldehyde in 0.1 M cacodylate buffer. The cells were rinsed in the same buffer with 6.8% sucrose and postfixed in 2% OsO4/3% K4Fe(CN)6 in 0.2 M cacodylate buffer at 4°C for 1 h. After rinsing in 0.1 M cacodylate buffer and dehydration in a graded alcohol series, the cells were embedded in Epon 812 and polymerized at 58°C. Finally, ultrathin sections (60 nm) were cut and stained with uranyl acetate and lead citrate. The sections were examined using a Philips CM 100 electron microscope operating at 80 kV, and micrographs were taken.
Determination of the TJ “Barrier” Function
To determine whether TJs restrict paracellular diffusion of solutes from the BC lumen to the basolateral medium, cells were incubated with 0.5 μM 5-carboxyfluorescein diacetate (CFDA; Sigma-Aldrich) at 37°C for 30 min to allow its internalization and subsequent translocation into the BC lumen by the multidrug resistance protein (MRP)2 ATP-binding cassette (ABC) transporter. After extensive washes, the capacity of BC to contain the fluorescent CFDA was analyzed with a fluorescence microscope.
To test the restriction of the passage of solutes from the culture medium to the BC lumen by TJs, the basolateral side of the cells was incubated with the fluid phase marker lucifer yellow at 100 μM (Sigma-Aldrich) on ice for 30 min, and, after extensive washes on ice, the absence or presence of fluorescence in the BC lumens was determined.
Determination of the TJ “Fence” Function
To examine the fence function of TJs, N-(N-[6-[(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]caproyl])-sphingosylphosphorylcholine (C6-NBD-SM) was used as described previously (van der Wouden et al., 2002). Briefly, cells were cooled to 4°C by washing with ice-cold HBSS, and incubated with 4 μM C6-NBD-SM for 30 min at 4°C to label the basolateral plasma membrane while preventing endocytosis. The cells were then observed under fluorescence microscopy to detect whether the fluorescent lipids had diffused from the basolateral surface domain to the BC surface membrane. In an alternative experiment, cells were incubated with C6-NBD-SM at 37°C for 30 min to allow endocytosis and subsequent transcytosis to the BC membrane (van IJzendoorn et al., 1997). To terminate transport, the cells were cooled by washing with ice-cold HBSS. Fluorescent lipids remaining at the basolateral surface was removed by a back-exchange procedure at 4°C as described in van der Wouden et al. (2002). The cells were incubated for another 15 min on ice and then examined under the fluorescence microscope to detect whether diffusion of the lipid probe had occurred from the apical to the basolateral surface.
Transcytosis Assay
Three-day-old HepG2 and HepG2-AJ− cells were washed with HBSS buffer and incubated in HBSS supplemented with antibodies against extracellular epitopes of DPPIV, MDR1, or 5′nucleotidase, at 4°C for 30 min. After extensive washes, the bound antibodies were chased at 37°C for 60 min. Cells were then fixed on ice and processed for immunofluorescence analyses as described above.
Protein Gel Electrophoresis and Western Blotting
HepG2 or HepG2-AJ− cells were washed and scraped in ice-cold KCl/HEPES buffer (140 mM KCl and 20 mM HEPES, pH 7.4), supplemented with a cocktail of protease inhibitors. The cells were homogenized with a tight douncer and centrifuged at 2000 rpm at 4°C for 10 min. The supernatant (cytosol and membranes) was centrifuged at 100,000 × g at 4°C for 30 min, and the pellet (membrane fraction) was resuspended in KCl/HEPES buffer with protease inhibitors. Twenty or 40 μg of membrane proteins were separated with SDS-polyacrylamide gel electrophoresis and subjected to Western blot analysis by using monoclonal antibodies against DPPIV, 5′-nucleotidase (5′NT), and MDR1 as described previously using an enhanced chemiluminescence (ECL) detection system (Aït Slimane et al., 2003). ECL-films were scanned, and protein bands were quantified using free Scion Image software (Scion, Frederick, MD).
RESULTS
HepG2-AJ−, a Mutant HepG2 Cell Line Lacking E-Cadherin/β-Catenin–based Adherens Junctions
We have obtained in our laboratory a human hepatocyte HepG2 cell line that displays a striking reduction in cell–cell adhesion strength compared with parental HepG2 cells. Detailed analyses revealed that in these mutant cells, β-catenin and E-cadherin are sequestered intracellularly. Consequently, β-catenin and E-cadherin do not form adherens junctions (Figure 1). Note that these cells do not necessarily remain as single cells and that they can engage in cell-to-cell interactions that are mediated by adhesive contacts other than adherens junctions (see below). Details of this mutant HepG2 cell line, which in this article is referred to as HepG2-AJ− cells, will be described in a separate study.
Figure 1.
The HepG2-AJ− cell line presents a defect in E-cadherin and β-catenin localization. The parental HepG2 and AJ-defective HepG2-AJ− cell lines were grown for 72 h, fixed, and immunolabeled for β-catenin (A) and E-cadherin (B). Arrows highlight the presence of β-catenin (A) and E-cadherin (B) at the cell–cell contact in parental HepG2 cells, whereas these proteins are retained intracellularly in HepG2-AJ− cells. Bar, 20 μm.
HepG2-AJ− Cells Develop Intercellular Apical Lumens
Parental HepG2 cells display a typical hepatocellular polarity characterized by the presence of an apical surface domain, representing the BC surface, which is enclosed by adjacent cells. BCs can be readily visualized by light and electron microscopy, and they typically occur as intercellular spherical structures containing numerous microvilli (van IJzendoorn et al., 1997; van IJzendoorn and Hoekstra, 2000).
We examined HepG2-AJ− cells with regard to their ability to develop apical BC surface domains. HepG2-AJ− cells were cultured as described in Materials and Methods for 3 d, fixed, and processed for microscopy. Light microscopical analysis of 1-μm-thick sections (Figure 2A) as well as transmission electron microscopical evaluation (Figure 2B) demonstrates that HepG2-AJ− cells developed intercellular BCs. The BCs in HepG2-AJ− cells were filled with microvilli (Figure 2B), confirming their apical nature. To further confirm the apical identity of the membranes lining the BCs, we examined the localization of proteins that are known to reside at the bile canalicular surface in vivo. The peripheral ERM-family protein radixin, the glycosylphosphatidylinositol (GPI)-anchored ectoenzyme 5′NT, and the multimembrane spanning ABC- and multidrug transporter MDR1 all localized exclusively to the BC (Figure 2C). This was also the case for other BC-associated proteins, including the single membrane-spanning protein DPPIV and the microvillar actin cross-linking protein villin (see below). Note that groups of cells interact with each other predominantly through areas of the cell surface close to the location of the apical surface domain (Figure 2C, arrows).
Figure 2.
The HepG2-AJ− cell line develops a polarized phenotype. (A and B) 1-μm-thick coupe from embedded HepG2-AJ− cells observed with light microscopy (A) and with transmission electron microscopy (B). Note the presence of intercellular microvilli-lined apical lumens (white arrows in A) despite a clearly perturbed cell–cell contact (cell boundaries are depicted by dashed lines). (C) The localization of different known resident apical proteins (radixin, 5′nucleotidase, and MDR1) is restricted to the plasma membranes lining the intercellular lumens (arrows) in HepG2-AJ− cells (corresponding phase contrast images are depicted in the top panels). Bars, 5 μm (A and C), and 2.5 μm (B).
To add a quantitative measure to apical polarity in HepG2-AJ− cells, cells were cultured for 72 h, fixed, and stained with a monoclonal antibody raised against the microvilli-associated protein villin, and 4′,6-diamidino-2-phenylindole (DAPI) to visualize the nuclei. We then determined by immunofluorescence microscopy the number of BCs and cells in several microscopical fields, and we expressed cell polarity as the ratio BC/100 cells (van IJzendoorn and Hoekstra, 1998). As depicted in Table 1, HepG2-AJ− cells had developed 25.8 ± 0.5 BCs/100 cells. In comparison, parental HepG2 cells developed 48.6 ± 2.0 BCs/100 cells. Together, the data indicate that cells lacking E-cadherin/β-catenin–based AJs are capable of developing apical bile canalicular lumens, albeit to a lesser extent.
Table 1.
Appearance of apical lumens in HepG2 and HepG2-AJ− cells
| HepG2 | HepG2-AJ− | |
|---|---|---|
| No. BC/100 cells | 48.6 ± 2.0 | 25.8 ± 0.5 |
HepG2 and HepG2-AJ− cells were cultured for 72 h, fixed with acetone at −20°C for 5 min, and immunostained with monoclonal anti-villin antibodies and the nuclear stain DAPI (see Materials and Methods). The ratio BC/100 cells was then determined from several fields (minimal 500 cells) and expressed as mean ± SD of at least two independent experiments carried out in duplicate.
Apical Lumens Occur Intracellularly before Their Exposure at the Extracellular Space in HepG2-AJ− Cells
Evidently, the formation of an intercellular apical lumen requires the participation of at least two cells, and, hence, cell–cell interactions. When examining cell polarity under phase contrast, it was observed that microvilli-lined lumens displayed a transient intracellular appearance in HepG2-AJ− cells at earlier time points. On prolonged culture, these lumens seem to move vectorially to the cell surface facing adjacent cells where they “dock” and eventually merge with the plasma membrane to establish a single intercellular lumen. Such intracellular lumens resemble the VAC described in epithelial cells cultured in low-calcium medium (Vega-Salas et al., 1987), and they typically arise in epithelial cells that are prevented from establishing cell–cell adhesion (Vega-Salas et al., 1988). To address the appearance of VACs and intercellular lumens (BCs) in a quantitative manner as a function of time, cells were cultured for 48 or 72 h and then they were fixed. Apical lumens were then identified by microvilli appearance under phase-contrast microscopy (Figure 3B) and categorized as either located intracellularly (VAC), docked to the cell surface, or located intercellularly (BC) (Figure 3A). In parental HepG2 cells, >75 and 80% of the identified apical structures were intercellular after 48 and 72 h, respectively (Figure 3B, 1 and 2, and C). Most of the remaining apical structures were categorized as docked and only few (<10%) intracellular lumens were observed. In striking contrast, in HepG2-AJ− cells, only 25 and 45% of the apical structures occurred intercellularly after 48 and 72 h of culture, respectively (Figure 3B, 3–5). Forty-five and 28% of the apical structures were categorized as intracellular after 48 and 72 h, respectively, and the remainder was categorized as docked (Figure 3C). These data indicate that apical lumens in HepG2-AJ− occur intracellularly before their exposure at the extracellular space.
Figure 3.
Apical lumens occur intracellularly before their exposure at the extracellular space in HepG2-AJ− cells. HepG2 and HepG2-AJ− cells were plated and fixed at different time points. Apical lumen structures (arrows) were detected with light microscopy and categorized as intracellular (VAC), docked, or intercellular (BC) (see schematic representation in A). (B) Apical lumen structures in 48-h-old HepG2 cells were predominantly intercellular, whereas, by contrast, many apical lumen structures were intracellular in 48-h-old HepG2-AJ− cells. (C) Quantification of the distribution of apical lumen structures that were immunolabeled for the microvilli protein villin (mean ± SD of at least 500 cells).
Figure 4.
Tight junctions are present and functional in HepG2-AJ− cells. The localization of the tight junction proteins ZO-1(A, arrow) and PAR3 (B, arrow) was assayed in 3-d old HepG2-AJ− cells. Corresponding phase contrast images are shown in the top panels. Bars, 20 μm. (C) HepG2-AJ− cells were observed in electron microscopy to detect the presence of tight junctions (arrows). Enlargement in the bottom panel clearly shows TJs and a desmosome (D). Bars, 500 nm (top) and 60 nm (bottom). (D) The gate function (see text) of the tight junctions was examined using CFDA, a fluorescent substrate of MRP2. The HepG2-AJ− cells were loaded 30 min at 37°C with 0.5 μM CFDA. Note the restriction of CFDA in the BC lumens (arrows). The corresponding phase-contrast image is depicted in the top panel. Bar, 20 μm. (E) To confirm the proper gate function of the TJ, HepG2-AJ− cells were incubated for 30 min at 4°C in presence of 100 μM lucifer yellow. Note that lucifer yellow does not have access to the BC lumens (arrows). The corresponding phase contrast image is depicted in the top panel. Bar, 10 μm. (F and G) The fence function (see text) of the tight junctions was tested using the fluorescent lipid C6-NBD-sphingomyelin. When incorporated in the basolateral plasma membrane at 4°C, the NBD-SM remains at the basolateral membrane, and no lateral diffusion to the apical domain is observed (F, 0 min vs. 15 min). Alternatively, when the fluorescent lipid is loaded into the exoplasmic leaflet of the BC membrane according to a previously described protocol (van IJzendoorn et al., 1997; see Materials and Methods), no lateral diffusion of the lipid probe to the basolateral domain is observed (G, 0 min vs. 15 min). Arrows indicate the localization of the BCs. Bars, 20 μm.
Figure 5.
HepG2-AJ− cells present a defect in bile canalicular lumen remodeling. Parental HepG2 and HepG2-AJ− cells were cultured for 5 d, fixed, and stained against phalloidin-tetramethylrhodamine B isothiocyanate (TRITC) to visualize the subapical F-actin meshwork (top). Nuclei are stained with Hoechst (bottom). Note the appearance of extensive multicellular apical lumens in parental HepG2 cells (cf. Herrema et al., 2006), whereas in HepG2-AJ− cells apical lumens mainly remain between two adjacent cells, and no apical lumen remodeling has occurred. Bar, 20 μm.
HepG2-AJ− Cells Form Tight Junctions with Functional Barrier and Fence Functions
The development of apical surface domains to which apical resident proteins exclusively localize prompted us to determine whether functional TJs develop in HepG2-AJ− cells. For this, the subcellular distribution of two well-established TJ proteins, ZO-1 and PAR3, was examined. In both HepG2 and HepG2-AJ− cells, a typical ZO-1 and PAR3 staining pattern, i.e., perpendicular to the basolateral–apical axis, bordering the apical plasma membrane, was observed (Figure 4, A and B). Moreover, electron microscopical analysis revealed the presence of electron-dense tight junction structures bordering the apical plasma membrane domain (Figure 4C, arrows). To verify whether these TJs were functional, we first examined their ability to spatially segregate fluids present in the BC lumen on the one hand and the basolateral medium on the other hand (i.e., “gate” or barrier function), and, second, their ability to prevent lateral diffusion of membrane lipids between apical and basolateral surface domains (fence function). The gate or barrier function was tested using CFDA, a fluorescent substrate of apical plasma membrane ABC transporters, which, after cellular loading and cleavage by intracellular esterases that render it membrane impermeable, is rapidly translocated by these transporters into the apical lumen. As shown in Figure 4D, CFDA was effectively retained in the apical lumen in between adjacent HepG2-AJ− cells, suggesting that functional TJs are present that prevent paracellular leakage from the apical to the basolateral compartment. HepG2-AJ− cells were also incubated with lucifer yellow, a water-soluble fluorescent dye commonly used to test epithelial monolayer permeability, at 4°C (to prevent endocytosis) for 30 min. As shown in Figure 4E, no lucifer yellow could be detected in apical lumens identified by phase-contrast microscopy, indicating that no paracellular transport of solute from the basolateral to the apical compartment occurred, and corroborating the existence of an efficient TJ barrier function in HepG2-AJ− cells.
The fence function of TJs was investigated using the fluorescent lipid analogue C6NBD-sphingomyelin. HepG2-AJ− cells were incubated with C6NBD-sphingomyelin at 4°C for 30 min, which allows the probe to incorporate into the basolateral plasma membrane without being endocytosed (van IJzendoorn et al., 1997, Ait Slimane et al., 2003). Cells were then washed and incubated for an additional 15 min in buffer at 4°C. As shown in Figure 4F, the presence of the lipid probe in the basolateral plasma membrane was observed, whereas no labeling of apical plasma membranes could be detected (arrows), indicating that diffusion of the lipid probe from the basolateral to the apical plasma membrane was prevented. In an additional experiment, HepG2-AJ− cells were incubated with C6NBD-sphingomyelin at 37°C for 30 min, which allows its incorporation into the basolateral plasma membrane and subsequent transcytosis to the apical surface (van IJzendoorn et al., 1997). Fluorescent lipids remaining in the basolateral surface were then depleted by a back exchange procedure at 4°C (see Materials and Methods), leaving the lipid probe only in the apical plasma membrane and intracellular vesicles. HepG2-AJ− cells were then incubated in buffer at 4°C for another 15 min. As shown in Figure 4G, fluorescent lipids were retained at the apical surface, and no fluorescent lipids were detected at the basolateral domain, suggesting that lipids do not diffuse from the apical to the basolateral domain. Together, these data show that HepG2-AJ− cells develop TJs with functional barrier and fence functions.
HepG2-AJ− Cells Present a Defect in Apical Lumen Remodeling
In the liver, hepatocytes are arranged into one-to-two-cell-thick plates that allow formation of a tubular bile canalicular network. When parental HepG2 are cultured for 5 d or longer, intercellular BC elongate and they are remodeled to form large multicellular canalicular lumens (Figure 5A; Herrema et al., 2006). In striking contrast, long-term cultures of HepG2-AJ− cells failed to undergo these morphological changes, and BCs remain as small spherical lumens in between two cells (Figure 5B). These data suggest that E-cadherin/β-catenin–based AJs are dispensable for initial apical–basolateral polarity development but that they may be required for subsequent remodeling of BC lumens into multicellular tubes.
Blocking E-Cadherin Function in Parental HepG2 Cells Mimics the Phenotype of HepG2-AJ− Cells
We next investigated cell polarity development in parental HepG2 cells when cultured in the presence of E-cadherin blocking antibodies. Culturing HepG2 cells for 48 h in the presence of E-cadherin blocking antibodies resulted in the intracellular accumulation of E-cadherin (Figure 6A). The absence of E-cadherin from the cell surface did not alter the subcellular localization of β-catenin, which remained at the plasma membrane, even at sites where the plasma membranes of neighboring cells did not physically interact (Figure 6B, inset). Regardless of the intracellular accumulation of E-cadherin, HepG2 cells cultured in the presence of E-cadherin blocking antibodies developed intercellular BC lumens (Figure 6A) that were bordered by ZO-1–positive TJs (Figure 6C), and they contained the apical surface-associated ERM protein radixin (Figure 6D). Moreover, BCs were surrounded by a dense actin meshwork as in the parental HepG2 (Figure 6E). These data support our findings in HepG2-AJ− cells that E-cadherin–based, and, consequently, AJ-based cell–cell adhesions are dispensable for the development of apical–basolateral cell polarity.
Figure 6.
E-cadherin blocking antibodies do not prevent the development of polarity in parental HepG2 cells. Parental HepG2 cells were cultured for 3 d in presence of E-cadherin blocking antibody. Cells were then fixed and stained for E-cadherin (A), β-catenin (B, enlargement shows detailed localization of the protein at the membrane), the TJ protein ZO-1 (C), the apical marker radixin (D), and the actin marker phalloidin-TRITC (E). The corresponding phase-contrast pictures are depicted. The arrows in C–E point to the apical lumens. Bar, 20 μm.
HepG2-AJ− Cells Display Altered Apical Plasma Membrane-directed Trafficking of Dipeptidyl Peptidase IV
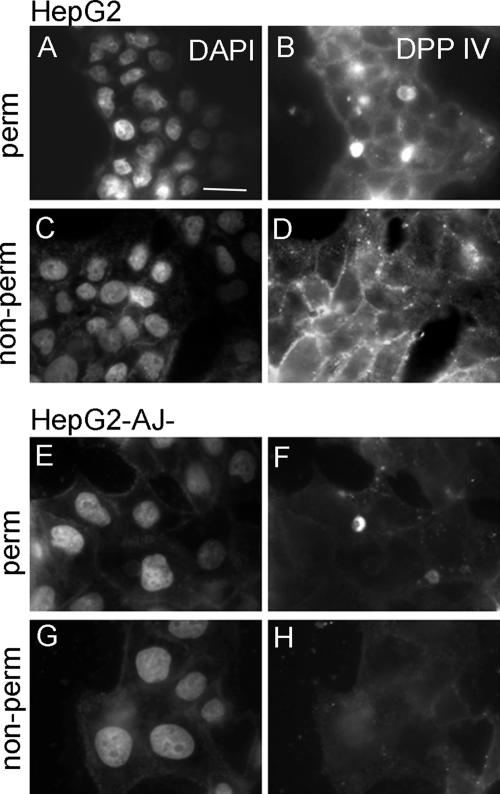
In hepatocytes, direct trafficking pathways from the Golgi apparatus to either apical or basolateral plasma membrane domains exist (Zaal et al., 1994). These pathways are selectively used by different apically destined proteins and lipids. Newly synthesized polytopic ABC transporters such as MDR1 and MRP2, and the copper transporter ATP7B, are targeted directly to the BC membranes (Sai et al., 1999; Kipp and Arias, 2000, 2002; Ait Slimane et al., 2003). In contrast, apical resident proteins such as the GPI-anchored protein 5′NT, and the single transmembrane ectoenzyme DPPIV, are first targeted to the basolateral surface, followed by their internalization and transcytosis to the BC membranes (Bartles et al., 1987; Schell et al., 1992; Ihrke et al., 1998; Bastaki et al., 2002; Ait Slimane et al., 2003). All basolateral proteins are presumed to be targeted to the basolateral surface directly (i.e., without prior delivery to the apical domain). We compared the trafficking itineraries of MDR1, 5′NT, and DPPIV between HepG2 and HepG2-AJ− cells to obtain information as to the possible involvement of E-cadherin–mediated cell–cell adhesion in these events. The steady-state distributions of MDR1 (Figure 2C) and 5′NT (Figure 2C) in HepG2-AJ− cells revealed extensive and almost exclusive localization of the proteins at the BC. DPPIV was abundantly present at the BC (Figure 7, A and B) and in the basolateral plasma membrane (Figure 7, C and D) of parental HepG2 cells, evidenced by immunostaining in permeabilized and nonpermeabilized cells, respectively. However, in HepG2-AJ− cells, DPPIV was only detected at the BC (Figure 7, E and F) and not at the basolateral surface (Figure 7, G and H), suggesting that in these cells DPPIV was delivered to the apical domain without prior basolateral delivery. To further investigate this, the basolateral surface of living HepG2 and HepG2-AJ− cells was exposed at 4°C for 30 min to antibodies against extracellular epitopes on DPPIV, followed by a wash and chase of the antibodies at 37°C for 60 min. Cells were then fixed on ice and processed for immunofluorescence analyses. Basolateral staining and basolateral-to-apical transcytosis was observed in HepG2 cells (Figure 8, A–D), consistent with its indirect trafficking in hepatocytes (Bastaki et al., 2002). However, in striking contrast, no basolateral staining and transcytosis of DPPIV was observed in HepG2-AJ− cells (Figure 8, E–H) despite its abundant apical localization at steady state (Figure 7, G–I), suggesting that delivery of DPPIV to the apical plasma membrane in HepG2-AJ− cells occurs via a direct pathway that does not involve passage through the basolateral domain. Western blot analysis revealed no significant difference in the expression level of DPPIV between HepG2 and HepG2-AJ− cells (Figure 8, I and J). Similar transcytosis assays were performed using antibodies raised against extracellular epitopes on MDR1 and 5′NT. In case of MDR1, we detected no basolateral staining (Figure 9A) or basolateral to apical transcytosis in HepG2-AJ− cells (Figure 9B), consistent with the reported direct trafficking of MDR1 from the Golgi apparatus to the BC in HepG2 cells (Kipp and Arias, 2000; Aït Slimane et al., 2003). With 5′NT, basolateral staining (Figure 9C) and basolateral-to-apical transcytosis (Figure 9D) were observed in HepG2-AJ− cells, similarly as reported in parental HepG2 cells (Aït Slimane et al., 2003). These data may suggest that E-cadherin–mediated cell–cell adhesions in hepatocytes direct the basolateral targeting of DPPIV but not that of other apical proteins such as GPI-anchored 5′NT (see Discussion). Moreover, for DPPIV these data suggest that, in contrast to previous suggestions, hepatocytes possess a Golgi-based machinery for directly sorting single transmembrane apical proteins such as DPPIV.
Figure 7.
Steady-state distribution of DPPIV in HepG2 and HepG2-AJ− cells. Three-day-old HepG2 (A–D) or HepG2-AJ− cells (E–H) were fixed and stained for DPPIV after permeabilization (A and B, E–F) or without permeabilization (C and D, G and H). Note the absence of basolateral staining of DPPIV in HepG2-AJ− cells. Bars, 10 μm.
Figure 8.
Transcytosis of DPPIV in HepG2 and HepG2-AJ− cells. The basolateral surface of HepG2 (A–D) and HepG2-AJ− cells (E–H) was exposed to antibodies against extracellular epitopes of DPPIV at 4°C for 30 min, followed by a wash and chase of the antibodies at 37°C for 60 min. Cells were fixed before (A and B, E and F) or after the chase (C and D, G and H) and stained for DPPIV. Phase-contrast pictures are shown on the left. Note the appearance of DPP IV in BC (arrows) after a 60-min chase in HepG2 cells, whereas no transcytosis of DPPIV occurs in HepG2-AJ− cells. (I) Expression levels of DPPIV, 5′NT, and MDR1 in HepG2 and HepG2-AJ− cells. (J) Quantitation of the relative mean expression levels of proteins from three immunoblots.
Figure 9.
Transcytosis of MDR1 and 5′NT. The basolateral surface of HepG2-AJ− cells was exposed to antibodies against extracellular epitopes of MDR1 (A) and 5′NT (C) at 4°C for 30 min, followed by a wash and chase of the antibodies at 37°C for 60 min (B and D, respectively). Note the absence of transcytosis of MDR1 (A and B; cf. Ait Slimane et al., 2003) and the appearance of 5′NT in BC after the 60 min chase (C and D). Bar, 20 μm.
DISCUSSION
In this study, we demonstrate that hepatic HepG2-AJ− cells develop functional tight junctions and apical–basolateral polarity in the absence of E-cadherin–mediated cell–cell adhesions. Earlier studies with cultured cells suggested that E-cadherin–dependent cell–cell adhesion is the founding event of epithelial polarity (for reviews, see Le Bivic et al., 2005; Miyoshi and Takai, 2005, and references herein). These studies were mostly based on monitoring the ordered recruitment of specific AJ and TJ proteins in time after disruption of the epithelial monolayer by calcium deprivation. Expression of a mutant of E-cadherin that lacks only the adhesive extracellular domain, however, was shown to increase TJ assembly, and it suggested that the hierarchical regulation of apical junction complexes is not absolute (Troxell et al., 2000). More recently, Baas et al. (2004) demonstrated that single intestinal epithelial cells that overexpress an inducible activator of LKB1/PAR4 develop an apical “brush-border” domain with apical proteins that are segregated from basolateral proteins by TJs, indicating polarity development without the need for prior E-cadherin–based cell–cell contact establishment. Harris and Peifer (2004, 2005) demonstrated that polarity of epithelial cells in early stages of Drosophila development can be established without formation of E-cadherin complexes. Capaldo and Macara (2007) recently showed that depletion of E-cadherin from kidney epithelial Madin-Darby canine kidney (MDCK) cells disrupts the establishment but not maintenance of apical–basolateral polarity and cell junctions. Our data, obtained in human hepatic cells, which, in contrast to cells used in other studies, do express endogenous wild-type E-cadherin but are incapable of establishing E-cadherin–based AJs, provide new evidence for and underscore the concept that E-cadherin function at the cell surface is dispensable for TJ formation and apical–basolateral polarity development per se.
Although apical–basolateral polarity establishment was evident, development of intercellular apical lumens in freshly plated HepG2-AJ− cell cultures was delayed, and microvilli-lined BCs were observed intracellularly before their appearance in between adjacent cells. This is consistent with earlier reports that showed that loss of cell–cell adhesion precludes the activation of a signaling cascade that is required for the efficient delivery of apical vesicles to the plasma membrane. Instead, apical vesicles accumulate in the cytoplasm, and, by means of homotypic fusion, give rise to intracellular VACs that functionally and structurally resemble intercellular apical lumens (Vega-Salas et al., 1987, 1988, 1993). In contrast to the cells in those studies, which lacked any type of cell–cell adhesion, intracellular apical lumens in HepG2-AJ− cells evidently dock with the cell surface and form functional intercellular apical lumens. Conceivably, a delay in the acquisition of cell–cell adhesion strength due to the lack of functional E-cadherin is responsible for the temporary impediment of intercellular apical lumen formation in HepG2-AJ− cells.
Whereas E-cadherin–mediated cell–cell adhesion seems dispensable for formation of functional TJ and apical–basolateral polarity with a correct orientation, it is not for subsequent apical lumen morphogenesis and remodeling. Thus, whereas prolonged culturing of parental HepG2 cells results in the development of elongated and multicellular apical lumens, this does not occur in HepG2-AJ− cell cultures, in which apical lumens remain as spherical lumens in between mostly two cells. Apical lumen remodeling in HepG2 cells is mediated by the deposition of extracellular matrix and subsequent inhibition of Rho kinase and myosin II signaling, which, in turn, are instrumental for cell multilayering and cell-to-cell reorientation or cell patterning (Herrema et al., 2006). Our data indicate that E-cadherin–regulated cell–cell adhesion is essential for dynamic cell-to-cell (re)orientation and apical lumen remodeling. Indeed, loss of E-cadherin in vivo prevents the development of epithelial tissues and is embryonically lethal (Johnson et al., 1986; Harris et al., 2005).
Interestingly, the indirect apical trafficking pathway of the single transmembrane protein DPPIV in HepG2 cells changes to a direct apical trafficking route in HepG2-AJ− cells. In contrast, the indirect and direct trafficking itineraries of the GPI-anchored 5′NT and the polytopic ABC transporters MRP2 and MDR1/3, respectively, are similar in HepG2 and HepG2-AJ− cells. In different epithelial cell lines, the resident apical membrane protein DPPIV can be targeted to both apical and basolateral surface but with a highly variable apical-to-basolateral ratio (Aït Slimane et al., 2001, and references herein). Thus, whereas in MDCK cells most DPPIV is transported directly to the apical domain (Casanova et al., 1991), in hepatocytes DPPIV is predominantly sorted to the basolateral domain, followed by its transcytosis to the apical surface (Bastaki et al., 2002; Aït Slimane et al., 2003). Consistent with the diversity in polarized trafficking, DPPIV has been proposed to contain both apical and basolateral sorting signals (Weisz et al., 1992), the dominance of which may be a reflection of competing vesicle targeting machineries (Zurzolo et al., 1992). In contrast to previous suggestions (Bastaki et al., 2002), our data indicate that hepatocytes do harbor the machinery for directly sorting DPPIV, and possibly other single transmembrane proteins, to the apical surface.
Two scenarios can be envisioned to account for the altered trafficking of DPPIV in HepG2-AJ− cells. First, the mutation in the HepG2-AJ− cell line itself may affect the trafficking of multiple cargos, including DPPIV and E-cadherin, to the lateral domain. Whereas E-cadherin is retained intracellularly (Figure 1), the lumenal apical trafficking signal in DPPIV (Weisz et al., 1992) could then mediate the protein's direct trafficking to the apical surface. HepG2-AJ− cells have a serine-10 to alanine substitution in the p27(Kip1) protein, which prevents phosphorylation at this residue. In HepG2-AJ− cells, we find that β-catenin interacts with p27 and is prevented from interacting with E-cadherin (Théard, Raspe, Kalicharan, Hoekstra, and S. van IJzendoorn, unpublished data). The coinciding retention of E-cadherin in intracellular sites resembling the endoplasmic reticulum (Figure 1) is in harmony with the proposed role of β-catenin acting as a chauffeur to facilitate the transport of E-cadherin out of the endoplasmic reticulum (Chen et al., 1999). Although there is no evidence in the literature for the involvement of β-catenin in the trafficking of DPPIV, we can formally not exclude that the p27 mutation, in parallel to inhibiting the trafficking of E-cadherin, somehow inhibits the basolateral trafficking of DPPIV. In the second scenario, the direct apical trafficking of DPPIV in HepG2-AJ− cells may be a consequence of the absence of E-cadherin–mediated AJs, suggesting that E-cadherin–mediated AJs overrule the direct apical trafficking of DPPIV in hepatic cells. As part of an underlying mechanism, E-cadherin may modulate exocyst-derived “targeting patches” on the lateral membrane, as has been proposed by Yeaman et al., (2004). The role of the exocyst in polarized trafficking in hepatocytes has not been examined and further studies are thus needed to investigate this. Strikingly, the initial basolateral trafficking of the GPI-anchored 5′NT remains unaltered like the direct trafficking of polytopic ABC transporters in HepG2-AJ− cells, indicating that any influence of E-cadherin on polarized membrane trafficking would be restricted to specific (classes of) proteins, and/or restricted to specific (basolateral surface-directed) transport pathways.
ACKNOWLEDGMENTS
We thank M. Raspe for technical assistance, Dr. K. Ito for expert assistance, Dr. I. Zuhorn for constructive discussions, and K. Klappe and Dr. J. Šmisterová for technical discussions and reagents. We thank Dr. M. Wheelock for providing the E-cadherin antibody and Dr. H. P. Hauri for the DPPIV antibody.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-11-1040) on April 11, 2007.
REFERENCES
- Aït Slimane T., Lenoir C., Bello V., Delaunay J. L., Goding J. W., Chwetzoff S., Maurice M., Fransen J. A., Trugnan G. The cytoplasmic/transmembrane domain of dipeptidyl peptidase IV, a type II glycoprotein, contains an apical targeting signal that does not specifically interact with lipid rafts. Exp. Cell Res. 2001;270:45–55. doi: 10.1006/excr.2001.5337. [DOI] [PubMed] [Google Scholar]
- Aït Slimane T., Trugnan G., van IJzendoorn S. C., Hoekstra D. Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains. Mol. Biol. Cell. 2003;14:611–624. doi: 10.1091/mbc.E02-08-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas A. F., Kuipers J., van der Wel N. N., Batlle E., Koerten H. K., Peters P. J., Clevers H. C. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J. Cell Biol. 1987;105:1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastaki M., Braiterman L. T., Johns D. C., Chen Y. H., Hubbard A. L. Absence of direct delivery for single transmembrane apical proteins or their “Secretory” forms in polarized hepatic cells. Mol. Biol. Cell. 2002;13:225–237. doi: 10.1091/mbc.01-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldo C. T., Macara I. G. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Mishumi Y., Ikehara Y., Hubbard A. L., Mostov K. E. Direct apical sorting of rat liver dipeptidylpeptidase IV expressed in Madin-Darby canine kidney cells. J. Biol. Chem. 1991;266:24428–24432. [PubMed] [Google Scholar]
- Charron A. J., Nakamura S., Bacallao R., Wandinger-Ness A. Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J. Cell Biol. 2000;149:111–124. doi: 10.1083/jcb.149.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T., Stewart D. B., Nelson W. J. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J. Cell Biol. 1999;144:687–699. doi: 10.1083/jcb.144.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 2004;167:135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T. J., Peifer M. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 2005;170:813–823. doi: 10.1083/jcb.200505127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrema H., Czajkowska D., Theard D., van der Wouden J. M., Kalicharan D., Zolghadr B., Hoekstra D., van Ijzendoorn S. C. Rho kinase, myosin-II, and p42/44 MAPK control extracellular matrix-mediated apical bile canalicular lumen morphogenesis in HepG2 cells. Mol. Biol. Cell. 2006;17:3291–3303. doi: 10.1091/mbc.E06-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrke G., Martin G. V., Shanks M. R., Schrader M., Schroer T. A., Hubbard A. L. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J. Cell Biol. 1998;141:115–133. doi: 10.1083/jcb.141.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. H., Maro B., Takeichi M. The role of cell adhesion in the synchronization and orientation of polarization in 8-cell mouse blastomeres. J. Embryol. Exp. Morphol. 1986;93:239–255. [PubMed] [Google Scholar]
- Kipp H., Arias I. M. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J. Biol. Chem. 2000;275:15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- Kipp H., Arias I. M. Trafficking of canalicular ABC transporters in hepatocytes. Annu. Rev. Physiol. 2002;64:595–608. doi: 10.1146/annurev.physiol.64.081501.155793. [DOI] [PubMed] [Google Scholar]
- Le Bivic A. E-cadherin-mediated adhesion is not the founding event of epithelial cell polarity in Drosophila. Trends Cell Biol. 2005;15:237–240. doi: 10.1016/j.tcb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- McNeill H., Ozawa M., Kemler R., Nelson W. J. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Miyoshi J., Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv. Drug Deliv. Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Sai Y., Nies A. T., Arias I. M. Bile acid secretion and direct targeting of mdr1-green fluorescent protein from Golgi to the canalicular membrane in polarized WIF-B cells. J. Cell Sci. 1999;112:4535–4545. doi: 10.1242/jcs.112.24.4535. [DOI] [PubMed] [Google Scholar]
- Schell M. Z., Maurice M., Stieger B., Hubbard A. L. 5′nucleotidase is sorted to the apical domain of hepatocytes via an indirect route. J. Cell Biol. 1992;119:1173–1182. doi: 10.1083/jcb.119.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Troxell M. L., Gopalakrishnan S., McCormack J., Poteat B. A., Pennington J., Garringer S. M., Schneeberger E. E., Nelson W. J., Marrs J. A. Inhibiting cadherin function by dominant mutant E-cadherin expression increases the extent of tight junction assembly. J. Cell Sci. 2000;113:985–996. doi: 10.1242/jcs.113.6.985. [DOI] [PubMed] [Google Scholar]
- van der Wouden J. M., van IJzendoorn S. C., Hoekstra D. Oncostatin M regulates membrane traffic and stimulates bile canalicular membrane biogenesis in HepG2 cells. EMBO J. 2002;21:6409–6418. doi: 10.1093/emboj/cdf629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn S. C., Hoekstra D. (Glyco)sphingolipids are sorted in subapical compartments in HepG2 cells: a role for non-Golgi-related intracellular sites in the polarized distribution of (glyco)sphingolipids. J. Cell Biol. 1998;142:683–696. doi: 10.1083/jcb.142.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn S. C., Zegers M. M., Kok J. W., Hoekstra D. Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J. Cell Biol. 1997;137:347–357. doi: 10.1083/jcb.137.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5:1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Gundersen D., Rodriguez-Boulan E. Formation of the apical pole of epithelial (Madin-Darby canine kidney) cells: polarity of an apical protein is independent of tight junctions while segregation of a basolateral marker requires cell-cell interactions. Cell Biol. 1987;104:905–916. doi: 10.1083/jcb.104.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., Salas P. J., Rodriguez-Boulan E. Exocytosis of vacuolar apical compartment (VAC): a cell-cell contact controlled mechanism for the establishment of the apical plasma membrane domain in epithelial cells. J. Cell Biol. 1988;107:1717–1728. doi: 10.1083/jcb.107.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Salas D. E., San Martino J. A., Salas P. J., Baldi A. Vacuolar apical compartment (VAC) in breast carcinoma cell lines (MCF-7 and T47D): failure of the cell-cell regulated exocytosis mechanism of apical membrane. Differentiation. 1993;54:131–141. doi: 10.1111/j.1432-0436.1993.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Weisz O. A., Machamer C. E., Hubbard A. L. Rat liver dipeptidylpeptidase IV contains competing apical and basolateral targeting information. J. Biol. Chem. 1992;267:22282–22288. [PubMed] [Google Scholar]
- Yeaman C., et al. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat. Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C., Grindstaff K. K., Nelson W. J. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Zaal K. J., Kok J. W., Sormunen R., Eskelinen S., Hoekstra D. Intracellular sites involved in the biogenesis of bile canaliculi in hepatic cells. Eur. J. Cell Biol. 1994;63:10–19. [PubMed] [Google Scholar]
- Zurzolo C., Le Bivic A., Quaroni A., Nitsch L., Rodriguez-Boulan E. Modulation of transcytotic and direct targeting pathways in a polarized thyroid cell line. EMBO J. 1992;11:2337–2344. doi: 10.1002/j.1460-2075.1992.tb05293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]