Abstract
Transforming growth factor β (TGFβ) plays a critical role in connective tissue remodeling by fibroblasts during development, tissue repair, and fibrosis. We investigated the molecular pathways in the transmission of TGFβ signals that lead to features of connective tissue remodeling, namely formation of an α-smooth muscle actin (α-SMA) cytoskeleton, matrix contraction, and expression of profibrotic genes. TGFβ causes the activation of focal adhesion kinase (FAK), leading to JNK phosphorylation. TGFβ induces JNK-dependent actin stress fiber formation, matrix contraction, and expression of profibrotic genes in fak+/+, but not fak−/−, fibroblasts. Overexpression of MEKK1, a kinase acting upstream of JNK, rescues TGFβ responsiveness of JNK-dependent transcripts and actin stress fiber formation in FAK-deficient fibroblasts. Thus we propose a FAK-MEKK1-JNK pathway in the transmission of TGFβ signals leading to the control of α-SMA cytoskeleton reorganization, matrix contraction, and profibrotic gene expression and hence to the physiological and pathological effects of TGFβ on connective tissue remodeling by fibroblasts.
INTRODUCTION
Normal tissue repair requires that fibroblasts migrate into the wound, where they synthesize, remodel, and contract extracellular matrix (ECM), resulting in wound closure. The type of fibroblast that executes this function is the myofibroblast, so-called because this cell type expresses α-smooth muscle actin (α-SMA), which is organized into stress fibers that exert contractile forces on the ECM through specialized cell surface structures called focal adhesions (Hinz and Gabbiani, 2003). In a properly healed wound, few myofibroblasts remain; however, if myofibroblasts persist in the lesion, scarring results (Desmouliere et al., 2005). Excessive scarring can lead to chronic fibrosis, which can result in organ failure and death. Fibrotic disease represents one of the largest groups of disease for which there is no therapy. Understanding the pathways that selectively influence myofibroblast differentiation and action is therefore essential in understanding not only in understanding the basis of normal tissue repair but also in appreciating how to control the progression of persistent fibrosis.
Transforming growth factor β (TGFβ) ligands play an important role in cell proliferation, lineage determination, extracellular matrix production, cell motility, apoptosis, and modulation of immune function (McCartney-Francis et al., 1998). TGFβ also induces ECM synthesis and remodeling and myofibroblast differentiation (Leask and Abraham, 2004). Exaggerated TGFβ signaling in fibroblasts contributes to chronic fibrosis (Chen et al., 2005, 2006). The intracellular signaling pathway downstream to the TGFβ receptors is mediated by the Smad family of transcription factors (Shi and Massague, 2003). Activation of the type I receptor results in phosphorylation of the pathway-restricted Smad2 and Smad3, which then form a heteromeric complex with Smad4. The complex translocates to the nucleus where, either alone or in association with a DNA-binding subunit, it activates target genes by binding to specific promoter elements (Zawel et al., 1998). Exaggerated TGFβ signaling is a hallmark of fibrotic diseases, such as scleroderma, and cooperation of TGFβ with proteins such as endothelin-1 or CCN2 is likely to result in persistent fibrosis (Leask, 2006; Shi-wen et al., 2006a,b). However, TGFβ is important for many processes; thus broad targeting of TGFβ, for example by using a TGFβ type I (ALK5) receptor or Smad antagonists is likely to have deleterious consequences (McCartney-Francis et al., 1998; Leask and Abraham, 2004).
It is now appreciated that this model of TGFβ signaling is simplistic. TGFβ activates non-Smad signaling pathways, including MAP kinase cascades such as ras/MEK/ERK and JNK, which appear to be required for expression of target genes in a promoter-specific manner (Leask and Abraham, 2004; Javelaud and Mauviel, 2005). However, the mechanism underlying the activation of these ancillary non-Smad pathways is unclear. It has also become apparent that adhesive molecules are involved with mediating TGFβ signals; for example, the extra domain A (EDA) form of fibronectin is required for the TGFβ induction of α-SMA in fibroblasts (Serini et al., 1998). In addition, TGFβ1-induced α-SMA expression in lung fibroblasts is blocked in nonadherent cells and in the presence of a FAK/src inhibitor (Thannickal et al., 2003). Adhesion to ECM involves integrins, whose signals are transmitted by focal adhesion kinase (FAK), a protein that is present at focal adhesions and is phosphorylated after integrin-mediated cell attachment (Parsons, 2003). FAK has been classically considered to mediate fibroblasts attachment to ECM; however, a priori it is also possible that FAK may be involved in transducing signals from growth factors as well (Cox et al., 2006). Indeed, the potential interplay between adhesive signaling cascades and cellular responses to growth factors remains poorly understood.
In this report, we use fibroblasts deficient in FAK to probe the contribution of FAK to signal transduction in response to TGFβ. We identify genes whose induction in fibroblasts is FAK-dependent and signaling pathways downstream of FAK required for TGFβ action in fibroblasts. Our results uncover new insights into the complex molecular mechanism underlying the contribution of adhesive signaling to growth factor responses in fibroblasts.
MATERIALS AND METHODS
Cell Culture and Harvesting
Embryonic fibroblasts taken from fak (focal adhesion kinase)+/+ and fak−/− mice (American Type Culture Collection, Manassas, VA) were grown in DMEM media containing 10% fetal calf serum, 2 mM l-glutamine, antibiotics (100 U/ml penicillin and 100 μg/ml streptomycin), and 1 mM sodium pyruvate (Invitrogen, Burlington, ON, Canada). Cells were grown at 37°C, 5% CO2, harvested at 90–95% confluence, and washed twice in phosphate-buffered saline (PBS). The cells were then scraped in PBS, and pellets were collected after centrifugation at 2000 rpm for 5 min at 4°C, resuspended in 2% SDS, quantified (BCA Kit, Pierce, Rockford, IL), and placed in Laemmli sample buffer containing complete protease and phosphates inhibitors cocktail (Boehringer Mannheim, Mannheim, Germany). Cells were used at passage 5. For bright-field microscopy, a Nikon Eclipse microscope was used (Mississauga, ON, Canada). Cell spreading (100 cells) was measured using Northern Eclipse (Empix, Mississauga, ON, Canada) software.
Western Blot Analysis
Equal amounts of protein (20 μg) were subjected to SDS-PAGE. Gels were electrophoretically transferred to nitrocellulose (Invitrogen). Membrane was blocked with 5% nonfat dry milk in Tris-buffered saline, 0.1% Tween 20 (Sigma, St. Louis, MO), and immunoblotting was performed using anti-phospho-JNK, anti-JNK, anti-FAK, or anti-phospho-FAK antibodies (Cell Signaling Technology, Beverly, MA), anti-type I collagen (Biodesign, Saco, ME) and anti-α-SMA (Sigma) antibodies as described by the manufacturer. Cells were pretreated with PP2 (10 μM, Calbiochem, 1 h) or DMSO when indicated. Anti-GAPDH (Sigma) antibodies were used as loading controls. Blots were then developed by incubation with biotinylated anti-rabbit or anti-mouse antibodies (1:1000; Vector Laboratories, Burlingame, CA) as secondary antibodies, followed by incubation with ABC regent (Vector Laboratories, Burlingame, CA). Signal was detected using a luminescence kit (ECL kit; Amersham, Little Chalfont, United Kingdom) and x-ray film. Densitometry was performed using Gel Base/Gel-Blot Pro (Synoptics, Cambridge, United Kingdom).
Cell Transfections
Transfections of fibroblasts were performed essentially as previously described (Holmes et al., 2001; Shi-wen et al., 2006b). Briefly 2 × 105 cells were seeded into each well of a six-well plate. The next day, cells were transfected using FuGene (Roche, Indianapolis, IN) in a ratio of 3 μl FuGene:2 μg DNA. Cells were transfected with either a vector encoding constitutively active MEKK1 (Stratagene, La Jolla, CA) or an empty expression vector. When indicated, cells were cotransfected with an expression vector encoding green fluorescent protein (GFP) under the control of the cytomegalovirus (CMV) promoter (CMV-GFP; Clontech, Palo Alto, CA). Cells were incubated after transfection for 24 h in serum-free media, followed by further incubation in the presence or absence of 4 ng/ml TGFβ1 (R&D Systems, Minneapolis, MN) in the presence or absence of SP600125 (10 μM, Calbiochem, La Jolla, CA) for 6 h. RNA was harvested and subjected to real-time PCR analysis.
RNA Quality Assessment, Probe Preparation, and Gene Chip Hybridization and Analysis
Microarrays and analysis were performed essentially as previously described (Shi-wen et al., 2004, 2006a, 2006b; Chen et al., 2005). All Gene Chips were processed at the London Regional Genomics Centre (Robarts Research Institute, London, ON, Canada; http://www.lrgc.ca). RNA was harvested (Trizol, Invitrogen) and quantified, and quality was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) and the RNA 6000 Nano kit (Caliper Life Sciences, Mountain View, CA). Quality data were then analyzed using the Degradometer (www.dnaarrays.org; mean degradation factor 1.99, SD 0.0678). Biotinylated complimentary RNA (cRNA) was prepared from 10 μg of total RNA as per the Affymetrix GeneChip Technical Analysis Manual (Affymetrix, Santa Clara, CA). Double-stranded cDNA was synthesized using SuperScript II (Invitrogen) and oligo(dT) 24 primers. Biotin-labeled cRNA was prepared by cDNA in vitro transcription using the Bizarre High-Yield RNA Transcript Labeling kit (Enzo Brioche, New York, NY) incorporating biotinylated UTP and CTP. Fifteen micrograms of labeled cRNA was hybridized to Mouse Genome 430 2.0 Gene Chips for 16 h at 45°C as described in the Affymetrix Technical Analysis Manual (Affymetrix). Gene Chips were stained with streptavidin-phycoerythrin, followed by an antibody solution and a second streptavidin-phycoerythrin solution, with all liquid handling performed by a GeneChip Fluidics Station 450. Gene Chips were scanned with the Affymetrix GeneChip Scanner 3000 (Affymetrix). Signal intensities for genes were generated using GCOS1.2 (Affymetrix) using default values for the Statistical Expression algorithm parameters and a target signal of 150 for all probe sets and a normalization value of 1. Normalization was performed in GeneSpring 7.2 (Agilent Technologies). The RMA preprocessor was used to import data from the. cel files. Data were first transformed (measurements < 0.01 set to 0.01) and then normalized per chip to the 50th percentile and per gene to the wild-type control samples. Experiments were performed twice, and fold changes were identified using the GeneSpring filter. Data presented in Table 1 are an average of these independent studies. The fold change between treated and untreated samples had to be at least twofold to identify a transcript as being altered. These criteria had to be met in both sets of experiments.
Table 1.
Genes whose induction in fibroblasts by TGFβ required FAK
| Affymetrix ID | Genbank ID | Fold increase WT | Fold increase KO | Gene name |
|---|---|---|---|---|
| Adhesion and matrix genes | ||||
| 1456344_at | BB003393 | 7 | 1 | Tenascin C |
| 1420753_at | NM_009390 | 7 | 1 | Tolloid-like 1 |
| 1423341_at | BB377873 | 4 | 1 | Chondroitin sulfate proteoglycan 4 |
| 1416318_at | AF426024 | 4 | 0.5 | Serine proteinase inhibitor member 1a |
| 1460227_at | BC008107 | 3 | 1 | Tissue inhibitor of metalloproteinase 1 |
| 1448291_at | NM_013599 | 3 | 1 | Matrix metalloproteinase 9 |
| 1458996_at | AI481717 | 3 | 1 | Integrin alpha 5 |
| 1419088_at | BI111620 | 3 | 1.5 | Tissue inhibitor of metalloproteinase 3 |
| 1446951_at | BB526042 | 2 | 1 | prolyl 4 hydroxylase |
| 1457823_at | BB533736 | 2 | 1.5 | cyr61 |
| 1451527_at | AF352788 | 3 | 1 | Procollagen C-endopeptidase enhancer 2 |
| 1450377_at | AI385532 | 2 | 1 | Thrombospondin 1 |
| Inflammatory genes | ||||
| 1450297_at | NM_031168 | 19 | 1 | Interleukin 6 |
| 1425832_a_at | AF301018 | 9 | 1 | Cxcr6 |
Average fold-increase in two independent microarray experiments is shown. Transcripts induced >2-fold in fak+/+ cells but nor fak−/− cells.
Real-Time PCR
Cells were serum-starved for 24 h and treated with 4 ng TGFβ for different lengths of time, as indicated. Total RNA was isolated using Trizol (Invitrogen), and the integrity of the RNA was verified by gel electrophoresis or Agilent bioanalyzer. For initial time-course analysis, total RNA (25 ng) was reverse-transcribed and amplified using TaqMan Assays on Demand (Applied Biosystems, Foster City, CA) in a 15-μl reaction volume containing two unlabeled primers and 6-carboxyfluoroscein–labeled TaqMan MGB probe. Samples were combined with TaqMan one-step mastermix (Applied Biosystems). Amplified sequences were detected using the ABI Prism 7900 HT sequence detector (Perkin Elmer-Cetus, Vaudreuil, QC, Canada) according to the manufacturer's instructions. Triplicate samples were run, and transcripts and expression values were standardized to values obtained with control 28 S RNA primers as previously described using the delta delta Ct method (Livak and Schmittgen, 2001; Shi-wen et al., 2006a,b). Statistical analysis was performed by the Student's paired t test. Less that 10% variation was seen within samples.
Floating Collagen Gel Cultures and Quantitation of Gel Contraction
Experiments were performed essentially as described (Shi-wen et al., 2004). Briefly, 24-well tissue culture plates were precoated with bovine serum albumin (BSA). Trypsinized fibroblasts were suspended in Molecular, Cellular, and Developmental Biology (MCDB) medium and mixed with collagen solution (one part of 0.2 M N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid [HEPES], pH 8.0; four parts collagen [Vitrogen-100, 3 mg/ml, Cohesion Technologies, Palo Alto, CA], and five parts of 2× MCDB) yielding a final concentration of 80,000 cells per ml and 1.2 mg/ml collagen. Collagen/cell suspension (1 ml) was added to each well. After polymerization, gels were detached from wells by adding 1 ml of MCDB medium. Contraction of the gel was quantified by loss of gel weight and decrease in gel diameter over a 24-h period. For inhibition experiments, cells were preincubated in the presence of inhibitor for 30 min before initiation of the assay. Comparison of collagen gel contraction was performed by using Student's unpaired t test. p < 0.05 was considered statistically significant.
Fibroblast-populated Collagen Lattices
Measurement of contractile force generated within a three-dimensional, tethered fibroblast-populated collagen lattice (FPCL) was performed as described previously (Eastwood et al., 1994; Shi-wen et al., 2004). Using 1 × 106 cells/ml collagen gel (First Link, Birmingham, United Kingdom), we measured the force generated across the collagen lattice with a culture force monitor that measures forces exerted by cells within a collagen lattice over 24 h as fibroblasts attach, spread, migrate, and differentiate into myofibroblasts. In brief, a rectangular fibroblast-seeded collagen gel was cast and floated in medium in 2% fetal calf serum in the presence or absence of TGFβ1 (4 ng/ml) or SP600125 (10 μM) while tethered to two flotation bars on either side of the long edges, in turn attached to a ground point at one end and a force transducer at the other. Cell-generated tensional forces in the collagen gel are detected by the force transducer and logged into a personal computer. Graphical readings are produced every 15 s, providing a continuous output of force (dynes: 1 × 10−5 N) generated (Eastwood et al., 1994). The cells used in these experiments were passage matched; experiments were run in parallel and three independent times. A representative trace is shown.
Cell Viability Assay
To determine cell viability, three independent assays were performed with three replicate samples. The MTT [3-(4,5-dimethyldiazol-2-yl)-2,5 diphenyl tetrazolium bromide] assay was performed on 12 h, with modifications of previously published methods (Kim et al., 2003). Briefly, fibroblast-populated gels were incubated with MTT solution (0.25 mg/ml in serum-free MCDB medium, 0.5 ml/gel) for 12 h. Alternatively, cells were cultured for 24 h in a monolayer. Gels were washed once with distilled water. Formazan crystals were then dissolved with dimethyl sulfoxide (500 μl/gel) by shaking 4–6 h at room temperature, and then absorbance at wavelength of 570 nm was determined with a microplate reader (Bio-Rad, Richmond, CA). Absolute OD value was obtained and expressed as percent of control.
Immunofluorescence Staining
Cells were seeded into 24-well plates containing glass coverslips, serum-starved for 24 h, and treated with 4 ng TGFβ for different lengths of time, as indicated. Cells were preincubated for 1 h in the presence or absence of SP600125 (10 μM) before addition of TGFβ. For staining of α-SMA and vinculin, cells were fixed with 4% paraformaldehyde in PBS for 15 min and then permeabilized with 0.2% (vol/vol) Triton X-100 in PBS for 5 min at room temperature. Cells were washed with PBS and then permeabilized with for 2–5 min. Cells were washed with PBS and then blocked with 1% (wt/vol) BSA in PBS for 1 h. Primary antibody, 100 μl, diluted in 1% BSA (wt/vol) in PBS was applied, and the cells were incubated for 1 h at room temperature. Primary antibodies were diluted as 1:200 dilution of a α-SMA antibody (Sigma), a 1:100 dilution of vinculin mAb (Sigma). Cells were washed for at least 20 min with PBS and then were incubated with a 1:100 dilution of Texas Red–labeled donkey anti-mouse secondary antibody in 1% (wt/vol) BSA in PBS for 1 h at room temperature. Cells were washed again for at least 20 min with PBS and then mounted in Vectashield mounting medium with DAPI (Vector Laboratories) before fluorescence microscopy. For double staining of phospho-JNK and vinculin, cells were fixed with 4% freshly diluted formaldehyde with PBS for 15 min at room temperature and then blocked with 5% goat serum in PBS containing 1% Triton X-100 for 1 h. Cells were washed the same as described above and incubated with anti-phospho-JNK (1:100 dilution, Cell Signaling) overnight at 4°C. Cells were washed and incubated with vinculin antibody (1:100) for 1 h at room temperature. Cells were then washed and incubated with a mixture of fluorescein isothiocyanate (FITC)-coupled goat anti-rabbit secondary antibody and Texas Red–labeled donkey anti-mouse secondary antibody (1:100 dilution) for 1 h at room temperature. Cells were then washed and mounted the same as above. Cells were viewed and photographed using Zeiss HB-100 fluorescence microscope (Toronto, ON, Canada). Fluorescence intensity of α-SMA fibers was quantified by line scan measurement using Northern Eclipse (Empix) software. Fluorescence intensity of vinculin-positive focal adhesions (FAs) was quantified by measuring 100 individual FA using Northern Eclipse (Empix) software.
RESULTS
FAK Is Required for the Ability of TGFβ To Induce JNK Phosphorylation in Fibroblasts
Previously, we have shown that the profibrotic protein endothelin-1 (ET-1) induces myofibroblast formation through a JNK-dependent mechanism and that constitutive ET-1–dependent JNK activation is a key feature of fibrotic fibroblasts (Shi-wen et al., 2006a). TGFβ can further induce JNK, leading to further ET-1 production in normal and fibrotic fibroblasts (Shi-wen et al., 2006a). TGFβ acts with ET-1 to induce a fibrotic phenotype (Shephard et al., 2004). To investigate the control mechanisms through which TGFβ can induce a fibrotic phenotype in fibroblasts, we wanted to evaluate to what extent TGFβ-induced JNK activation contributed to the induction of a tissue-remodeling phenotype in fibroblasts and to further investigate the mechanism underlying TGFβ-induced JNK activation.
We first used fibroblasts cultured from wild-type mice to verify, using Western blot analysis with anti-phospho-JNK and anti-JNK antibodies, that TGFβ could induce JNK in fibroblasts (Figure 1A). Enhanced adhesive signaling is a feature of fibrotic cells (Mimura et al., 2005; Chen et al., 2005). To assess if FAK was required for TGFβ to induce JNK, fibroblasts cultured from wild-type or fak−/− mice were serum-starved for 18 h and treated with or without TGFβ1. The resultant protein extracts were subjected to Western blot analysis with anti-phospho-JNK and anti-JNK antibodies. TGFβ-induced JNK phosphorylation was significantly impaired in fak−/− MEFs (Figure 1A). In the absence of TGFβ, fak−/− fibroblasts appear smaller and less spread than fak+/+ fibroblasts (Figure 2A). Using a standard assay, no difference in cell viability was observed between fak+/+ and fak−/− cells (Figure 2B). Indirect immunofluorescence analysis with anti-phospho-JNK antibody revealed that, whereas in wild-type fibroblasts TGFβ caused phospho-JNK epitopes to appear throughout the cell including at the cell periphery (Figure 1B, arrow), addition of TGFβ to fak−/− fibroblasts did not alter the localization of phospho-JNK epitopes (Figure 1B). In wild-type cells, TGFβ-induced FAK phosphorylation and inhibition of FAK/src with PP2 impaired the ability of TGFβ to induce JNK phosphorylation (Figure 1C). These results suggested that, in fibroblasts, TGFβ induces JNK in a FAK-dependent mechanism.
Figure 1.
TGFβ induces JNK phosphorylation in fibroblasts: requirement for FAK. (A) TGFβ1 activates FAK and JNK phosphorylation in fak+/+ cells. Fibroblasts from fak+/+ and fak−/− mice were serum-starved and treated with or without TGFβ1 (4 ng/ml) for 30 min. Cell extracts were prepared and subjected to Western blot analysis with anti-phospho-JNK1, anti-total JNK1 antibodies, anti-phospho-FAK, and anti-FAK antibodies. (B) TGFβ1 results in redistribution of phospho-JNK epitopes in fak+/+ cells. Fibroblasts from fak+/+ and fak−/− mice were treated with TGFβ for 30 min, fixed in paraformaldehyde, and stained with anti-phospho-JNK antibody followed by FITC-conjugated secondary antibody (green stain). Cells were also stained with anti-vinculin antibody followed by Texas red–conjugated secondary antibody (to detect the cell periphery, red stain). Cells were counterstained with DAPI (to detect the nucleus, blue stain). Note that addition of TGFβ to fak+/+ cells resulted in the appearance of phospho-JNK epitopes throughout the cell, including at the cell periphery (arrow). Phospho-JNK staining remains perinuclear in fak−/− cells. Please also note that a 30-min treatment with TGFβ was insufficient to cause appearance of “supermature” FA in fak+/+ cells (C) The FAK/src inhibitor PP2 reduces TGFβ1-induced JNK phosphorylation in fak+/+ cells. Fibroblasts from fak+/+ were serum-starved and treated with or without TGFβ1 (4 ng/ml) for 30 min. Cells were pretreated with DMSO or PP2 (10 μM), as indicated. Cell extracts were prepared and subjected to Western blot analysis with anti-phospho-JNK1 and anti-total JNK1 antibodies or anti-phospho-FAK or anti-FAK antibodies as indicated.
Figure 2.
MEFs deficient in FAK are smaller and less spread than fak +/+ MEFs. (A) Fibroblasts from wild-type (WT) and fak−/− (KO) mice were cultured and subjected to bright-field microscopy. Photographs were taken, and cell area (μm) was calculated using Northern Eclipse image analysis software. Average ± SD is shown (100 cells total/cell type). (B) A standard cell viability (MTT) assay was used to show that no significant difference in cell proliferation over 24 h was observed between WT and KO cells (n = 3; average ± SD is shown).
TGFβ-induced Myofibroblast Formation and Matrix Contraction Requires JNK and Is Impaired in fak−/− Fibroblasts
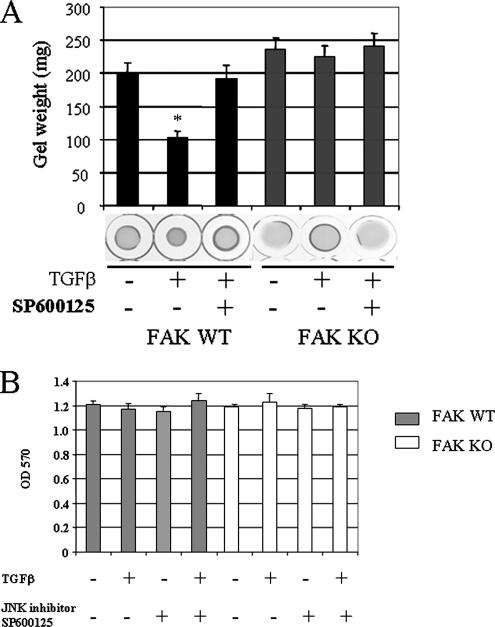
We then wanted to examine the contribution of FAK-dependent JNK activation on the ability of TGFβ to induce a tissue remodeling phenotype in fibroblasts. To begin to address this question, we assessed the dependence of TGFβ-induced myofibroblast formation on JNK and FAK. To perform this experiment, we treated wild-type fibroblasts with or without TGFβ for 24 h and subjected cells to indirect immunofluorescence analysis with an anti-α-SMA antibody. TGFβ potently increased α-SMA stress fiber formation in fak+/+ fibroblasts; yet a 45-min pretreatment of cells with the JNK inhibitor SP600125, before addition of TGFβ, caused a significant reduction in the appearance of α-SMA stress fibers (Figure 3A). Image analysis of individual fibroblasts revealed that amplitude and frequency of fluorescence intensity across cells, corresponding to individual stress fibers, were enhanced in fak+/+ cells but were reduced by JNK inhibition (Figure 3B). Consistent with the FAK-dependence of TGFβ-induced JNK activation in fibroblasts, TGFβ was relatively ineffective at inducing α-SMA stress fiber formation in fak−/− fibroblasts (Figure 3, A and B). Similarly, the ability of wild-type fibroblasts to contract a floating collagen gel lattice (Grinnell, 2003) in response to TGFβ is suppressed by JNK inhibition of wild-type fibroblasts (Figure 4A). Using a standard assay, fak+/+ and fak−/− cells showed equal cell viability both in the presence and absence of SP600125 under our experimental conditions (Figure 4B). Consistent with the FAK dependence of TGFβ-induced JNK activation in fibroblasts, TGFβ was relatively ineffective at inducing contraction of a collagen gel in fak−/− fibroblasts (Figure 4). Similar results were obtained using FPCL assays in which the generation of contractile forces across a tethered collagen gel lattice was examined (Figure 5).
Figure 3.
TGFβ induces α-SMA stress fiber formation in fibroblasts: requirement for JNK and FAK. (A) Indirect immunofluorescence analysis. Fibroblasts from wild-type (WT) and fak−/− (KO) mice were serum-starved and treated with or without TGFβ1 (4 ng/ml) for 24 h in the presence or absence of the JNK inhibitor SP600125 (SP). Cells were then fixed in paraformaldehyde and stained with α-SMA antibody followed by Texas red–conjugated secondary antibody. (B) Fluorescence intensity analysis. Cells obtained in A were analyzed using Northern Eclipse software by measuring fluorescence intensity across the cells, perpendicular to the α-SMA stress fibers. Intensity corresponds to individual α-SMA stress fibers. Note that JNK inhibition significantly impairs the formation of α-SMA stress fibers in normal fibroblasts and that the resultant cells are similar in appearance to fak−/− fibroblasts.
Figure 4.
TGFβ induces collagen gel contraction in fibroblasts: requirement for JNK and FAK. Floating gel contraction assay. (A) Fibroblasts from wild-type (WT) and fak−/− (KO) mice were placed within collagen gel lattices. After polymerization, lattices were detached from tissue culture plates, treated with or without TGFβ1 (4 ng/ml) for 24 h in the presence or absence of the JNK inhibitor SP600125 (SP). Contraction was monitored by measuring gel weight. *Significantly different from untreated control WT cells (p < 0.05). (B) A standard cell viability (MTT) assay was used to show that no significant difference in cell proliferation within the collagen gels was observed between WT and KO cells in the presence or absence of TGFβ or the JNK inhibitor (n = 3; average ± SD is shown).
Figure 5.
TGFβ induces collagen gel contraction in fibroblasts: requirement for JNK and FAK. FPCL assay. Fibroblasts from wild-type (WT) and fak−/− (KO) mice were placed within collagen gel lattices. After polymerization, lattices were detached from tissue culture plates and treated with or without TGFβ1 (4 ng/ml) for 24 h in the presence or absence of the JNK inhibitor SP600125 (SP). Contraction force generated across the gel was measured.
Supporting the notion that the ability of TGFβ to induce myofibroblast formation in fibroblasts was impaired in the absence of FAK, appearance of so-called “supermature” highly vinculin-positive FAs was impaired in fak−/− fibroblasts (Figure 6, A and B). Fewer FAs per cell were observed in fak−/− cells; however, TGFβ treatment of either fak+/+ or fak−/− cells did not result in a significant increase in FA number in either cell type (Figure 6C).
Figure 6.
TGFβ is unable to induce “supermature” highly vinculin-positive focal adhesions (FA) in fak−/− fibroblasts. (A) Immunofluorescence analysis. Fibroblasts from wild-type (WT) and fak−/− (KO) mice were serum-starved and treated with or without TGFβ1 (4 ng/ml) for 24 h. Cells were then fixed in paraformaldehyde and stained with anti-vimentin antibody followed by Texas red–conjugated secondary antibody. Note appearance of intense vinculin-positive staining at the cell periphery of WT cells treated with TGFβ (arrow, corresponding to supermature FAs). (B) Quantification of supermature FA. Image analysis software was used to measure the intensity of vinculin staining per FA (average fluorescence intensity) in cells photographed for A. The average intensity ± SD for 100 FA is shown. *Significantly different from untreated control WT cells (p < 0.05). (C) Quantification of number of FAs per cell. WT cells possess more FAs (number of vinculin dots) per cell than fak−/− (KO) cells (100 cells, average number of FAs/cell ± SD) is shown. Note that TGF|gb treatment of either WT or KO cells did not significantly increase the number of FAs/cell.
TGFβ-induced Expression of Profibrotic Genes Requires JNK and Is Impaired in fak−/− Fibroblasts
To assess the extent to which JNK activation was required for the ability of TGFβ to induce a matrix remodeling phenotype in fibroblasts, we assessed whether JNK was required for TGFβ to induce type I collagen and α-SMA mRNA and protein production in wild-type fibroblasts. We found that although TGFβ was able to induce α-SMA and type I collagen protein production in wild-type fibroblasts, a 45-min pretreatment with SP600125 before addition of TGFβ resulted in a reduction in α-SMA and type I collagen expression (Figures 7). A standard cell viability assay was used to show that SP600125 was not toxic to fibroblasts (not shown). As visualized by Western blot analysis, fak+/+ and fak−/− MEFs did not differ in their production of type I collagen or α-SMA in the absence of added TGFβ1 (Figure 7). Consistent with the FAK dependence of TGFβ-induced JNK activation in fibroblasts, TGFβ was relatively ineffective at inducing type I collagen or α-SMA protein in fak−/− fibroblasts (Figure 7).
Figure 7.
TGFβ induces type I collagen and α-SMA proteins in fibroblasts: requirement for JNK and FAK. Fibroblasts from wild-type (WT) and fak−/− (KO) mice were serum-starved and treated with or without TGFβ1 (4 ng/ml) for 24 h in the presence and absence of SP600125. Proteins were subjected to Western blot analysis with anti-type I collagen, anti-α-SMA, and anti-GAPDH antibodies, as indicated. Note that JNK inhibition reduced α-SMA and type I collagen induction in response to TGFβ in WT cells. Also, please note that induction of α-SMA and type I collagen protein did not occur in fak−/− cells.
To further evaluate the contribution of JNK and FAK to the ability of TGFβ to induce gene expression by MEFs, we cultured fak +/+ and fak−/− MEFs until 80% confluence and serum-starved cells for 24 h. Cells were then treated in the presence or absence of TGFβ (4 ng/ml) for an additional 6 h. Total RNA was prepared from these cells, reverse-transcribed, and applied to Affymetrix MOE430 arrays. Experiments were performed twice, and average induction values were obtained. Analysis of data by Genespring revealed TGFβ induced 942 transcripts greater than twofold in fak+/+ fibroblasts. Of these 304 were not induced greater than twofold in fak−/− fibroblasts. A representative selection of profibrotic transcripts is shown in Table 1. That the majority of TGFβ-induced genes in fak+/+ were also induced in fak−/− MEFs supported the notion that FAK was not generally required for the ability of TGFβ to induce gene expression. Profibrotic (adhesion, contraction, matrix) genes, as revealed by cluster analysis, were revealed to be both dependent on FAK included thrombospondin 1 (tsp1), integrin α5 (intα5), and tenascin C (Table 1). Results obtained using microarray analysis for these transcripts were results were verified using real-time PCR analysis of RNA isolated from fak+/+ and fak−/− MEFs treated with and without TGFβ for 6 h (Figure 8). The ability of TGFβ to induce vinculin mRNA was not impaired in fak−/− fibroblasts, confirming the selectivity of FAK action in conferring TGFβ responses to fibroblasts (Figure 8). Collectively, these results suggest that FAK is required for a subset of TGFβ responses in fibroblasts, including the induction of a cohort of profibrotic genes.
Figure 8.
TGFβ is unable to induce expression of a cohort of profibrotic mRNAs in fak−/− fibroblasts. Fibroblasts from wild-type (WT) and fak−/− (KO) mice were serum-starved and treated with or without TGFβ1 (4 ng/ml) for 6 h. mRNAs were harvested and subjected to real-time PCR analysis with primers detecting type I collagen, α-SMA, thrombospondin-1, integrin α5, tenascin C, and vinculin. Samples were standardized to GAPHH. Fold-increase in response to TGFβ is shown. Note that vinculin was responsive to TGFβ in wild-type (WT) and fak−/− (KO) cells. Note that SD within samples was <10%. *Significantly different fold-induction in response to TGFβ in KO cells compared with WT cells (p < 0.05).
Overexpression of MEKK1 Rescues the TGFβ-mediated Induction of mRNAs in fak−/− Fibroblasts
Having established the necessity for JNK in TGFβ responses in wild-type fibroblasts and that FAK was required for JNK activation, we then sought to provide a further illustration that the inability of fak−/− fibroblasts to support TGFβ responses was due to defects in the JNK signaling cascade. The kinase MEKK1 is upstream of JNK (Xia et al., 1998). Thus, to rescue the TGFβ-responsiveness defects of fak−/− fibroblasts, we transfected into this cell type either an expression vector encoding MEKK1 or an empty expression vector. Twenty-four hours after transfection, we treated cells in the presence of absence of TGFβ for 6 h. RNAs were harvested and subjected to real-time PCR analysis. We found that, compared with transfection empty expression vector, transfection of expression vector encoding MEKK1 rescued the TGF responsiveness of α-SMA, COL1A1, tsp-1, integrinα5, and tenascin C transcripts in fak−/− MEFs, but had no significant impact on vinculin induction (Figure 9). These results collectively are consistent with the notion that FAK-dependent JNK activation is essential for the ability of TGFβ to signal in fibroblasts, and, in particular, in TGFβ-induced fibrogenic responses.
Figure 9.
Transfection of MEKK1 expression vector restores the ability of TGFβ is to induce expression of a cohort of profibrotic mRNAs in fak−/− fibroblasts. Fibroblasts from fak−/− (KO) mice were transfected with empty expression vector or expression vector encoding activated MEKK1. Cells were serum-starved for 18 h after transfection and treated with or without TGFβ1 (4 ng/ml) for 6 h. mRNAs were harvested and subjected to real-time PCR analysis with primers detecting type I collagen, α-SMA, thrombospondin-1, integrin α5, tenascin C, or vinculin. Samples were standardized to GAPDH. Fold-increase in response to TGFβ is shown. Note that SD within samples was < 10%. *Significantly different fold-induction in response to TGFβ in KO cells transfected with activated MEKK1 compared with empty expression vector (p < 0.05).
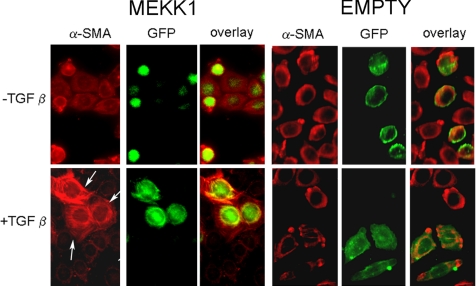
Furthermore, compared with transfection empty expression vector, transfection of expression vector encoding MEKK1 rescued the ability of TGFβ to induce α-SMA stress fibers (Figure 10). Collectively, these results are consistent with the notion that, in fibroblasts, FAK is required for the ability of TGFβ to signal through MEKK1/JNK and consequently to induce a matrix remodeling phenotype formation (Figure 11).
Figure 10.
Transfection of MEKK1 expression vector restores the ability of TGFβ is to induce α-SMA stress fibers in fak−/−fibroblasts. Fibroblasts from fak−/− (KO) mice were cotransfected with empty expression vector or expression vector encoding activated MEKK1 together with an expression vector encoding GFP. Cells were serum-starved for 18 h after transfection and treated with or without TGFβ1 (4 ng/ml) for 12 h. Cells were subjected to indirect immunofluorescence analysis using an anti-α-SMA antibody and an appropriate secondary antibody. Transfected cells were detected (using the GFP tag, arrow). Overexpression of MEKK1 restored the ability of TGFβ to induce α-SMA stress fibers.
Figure 11.
Summary of the contribution of FAK and JNK to a matrix remodeling phenotype in fibroblasts. TGFβ activates FAK, which is required for JNK activation. JNK is, in return, required for the induction of α-SMA stress fiber organization, matrix contraction, and induction of expression of a cohort of profibrotic gene.
DISCUSSION
In this study, we have examined the signaling events downstream of TGFβ, leading to the induction of an ECM-remodeling phenotype. In vitro and in vivo studies have consistently shown that application of TGFβ promotes ECM production and contraction, leading to granulation tissue deposition and the promotion of scarring (Roberts et al., 1986; Mori et al., 1999). Prior reports have focused on general TGFβ signaling pathway, including TGFβ receptors and Smads, in the production and remodeling of ECM (Verrecchia and Mauviel, 2002; Verrecchia et al., 2006). However, it is now appreciated that selective modulation of profibrotic signaling downstream of TGFβ would be beneficial in promoting and controlling the wound healing and scarring while leaving other effects of TGFβ unaltered (Leask et al., 2003; Leask and Abraham, 2004; Chen et al., 2006).
An increasing body of evidence supports the role of signaling through MAP kinase cascades in driving tissue repair and fibrogenesis; for example, the ras/MEK/ERK cascade controls the expression of TGFβ target genes in fibroblasts in a promoter-specific manner (Chen et al., 2002; Stratton et al., 2002; Leask et al., 2003). The JNK cascade has also been appreciated to mediate TGFβ responses in fibroblasts, yet the overall effect and biological significance of this cascade on TGFβ signaling in fibroblasts is unclear and in fact controversial. Fibroblasts deficient in JNK make excess TGFβ (Ventura et al., 2004), and overexpression of the JNK target c-jun blocks induction of a generic Smad3-dependent reporter (Verrecchia et al., 2001; Leask et al., 2003). Conversely, TGFβ induces α-SMA in a JNK-dependent manner (Hashimoto et al., 2001) and c-jun augments TGFβ-induction of 12-O-tetradecanoyl-13-acetate (TPA)-responsive gene promoter (TRE) elements that contain AP-1 sites (Zhang et al., 1998).
In this report, we investigate the contribution of JNK to TGFβ signaling in fibroblasts and the mechanism underlying the induction of this kinase. Previously, we have shown that JNK mediates the TGF induction of the ET-1 and that constitutive JNK activation, mediated by endogenous ET-1 production, at least partially contributes to the persistent fibrotic phenotype of scleroderma lung fibroblasts (Shi-wen et al., 2006a). However, the mechanism underlying JNK activation in response to TGFβ in normal fibroblasts is unclear. Furthermore, although it has been previously shown that TGF induction of α-SMA protein requires FAK and ED-A fibronectin (Serini et al., 1998; Thannickal et al., 2003), until this report the possibility that adhesive signaling in response to TGFβ may be required for induction of a matrix remodeling phenotype had yet to be tested. In this report, we show that although application of TGFβ to wild-type fibroblasts results in JNK phosphorylation, deletion of FAK resulted in a failure of fibroblasts to support not only JNK activation in response to TGFβ, but also the induction of a subset of TGFβ-induced genes including a cohort of profibrotic mRNAs, including type I collagen and α-SMA, as well as an α-SMA stress fiber network and ECM contraction. All of these responses required JNK in wild-type fibroblasts. Overexpression of constitutively active MEKK1, a kinase upstream of JNK, rescued the gene expression defects observed in fak−/− fibroblasts. Overall, these results suggest that FAK is required to mediate the induction of JNK by TGFβ in fibroblasts, resulting in the appearance of a tissue-remodeling phenotype. The TGFβ/FAK cascade may be further modified by matricellular proteins such as CCN2 (Shi-wen et al., 2006b; Leask and Abraham, 2006; Kennedy et al., 2007) to enhance tissue-remodeling responses.
It should be pointed out that in addition to mediating signaling events, FAK also provides other functions to cells such as providing a scaffold for the assembly of FA components and the cytoskeleton (Schaller, 2004). Thus loss of FAK expression in fibroblasts may affect these features as well. As an example, the differences between fak−/− and fak+/+ in terms of α-SMA protein seem more pronounced than the differences in α-SMA mRNA, indicating that FAK may also promote the stability of α-SMA protein within cells. However, our data revealing that overexpression of MEKK1 can at least partially restore the appearance of α-SMA stress fibers in response to TGFβ indicate that these additional features are not absolutely required for the MEKK1/JNK cascade to promote stress fiber formation. That said, it is possible that the FAK requirement is generic rather than specific, because FAK is required for normal cell attachment and spreading; indeed, previous findings demonstrating a requirement for EDA-FN and tension in TGFβ-stimulated formation of myofibroblasts (Serini et al., 1998; Dugina et al., 2001; Thannickal et al., 2003) are consistent with this notion.
In conclusion, we have provided evidence that the ability of TGFβ to induce a matrix-remodeling phenotype in fibroblasts depends on FAK/JNK. These results also suggest that a cross-talk exists between adhesive signaling cascades and TGFβ signaling in fibroblasts and that, compared with general blockade of TGFβ signaling by antagonizing TGFβ receptors or Smads, modifying FAK or JNK activity may be more appropriate in controlling normal tissue repair and in developing selective antifibrotic therapies.
ACKNOWLEDGMENTS
This work is funded by the Canadian Institute of Heath Research, the Canadian Foundation for Innovation, the Arthritis Research Campaign, the Reynaud's and Scleroderma Association and the Scleroderma Society. A.L. is an Arthritis Society (Scleroderma Society of Ontario) New Investigator and a recipient of Early Researcher Award. D.P. is the recipient of an Ontario Graduate Scholarship in Science and Technology. L.K. is the recipient of a Natural Sciences and Engineering Council Postgraduate Scholarship.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1121) on April 4, 2007.
REFERENCES
- Chen Y., Blom I. E., Sa S., Goldschmeding R., Abraham D. J., Leask A. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62:1149–1159. doi: 10.1111/j.1523-1755.2002.kid567.x. [DOI] [PubMed] [Google Scholar]
- Chen Y., et al. Matrix contraction by dermal fibroblasts requires transforming growth factor-beta/activin-linked kinase 5, heparan sulfate-containing proteoglycans, and MEK/ERK: insights into pathological scarring in chronic fibrotic disease. Am. J. Pathol. 2005;67:1699–1711. doi: 10.1016/s0002-9440(10)61252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Shi-wen X., Eastwood M., Black C. M., Denton C. P., Leask A., Abraham D. J., et al. Contribution of activin receptor-like kinase 5 (transforming growth factor beta receptor type I) signaling to the fibrotic phenotype of scleroderma fibroblasts. Arthritis. Rheum. 2006;54:1309–1316. doi: 10.1002/art.21725. [DOI] [PubMed] [Google Scholar]
- Cox B. D., Natarajan M., Stettner M. R., Gladson C. L. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J. Cell Biochem. 2006;99:35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- Desmouliere A., Chaponnier C., Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- Dugina V., Fontao L., Chaponnier C., Vasiliev J., Gabbiani G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J. Cell Sci. 2001;114:3285–3296. doi: 10.1242/jcs.114.18.3285. [DOI] [PubMed] [Google Scholar]
- Eastwood M., McGrouther D. A., Brown R. A. Fibroblast responses to mechanical forces Biochim. Biophys. Acta. 1994;1201:186–192. doi: 10.1016/0304-4165(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Gon Y., Takeshita I., Matsumoto K., Maruoka S., Horie T. Transforming growth Factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am. J. Respir. Crit. Care Med. 2001;163:152–157. doi: 10.1164/ajrccm.163.1.2005069. [DOI] [PubMed] [Google Scholar]
- Hinz B., Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003;14:538–546. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Holmes A., Abraham D. J., Sa S., Shiwen X., Black C. M., Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J. Biol. Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Javelaud D., Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- Kennedy L., Liu S., Shi-Wen X., Chen Y., Eastwood M., Carter D. E., Lyons K. M., Black C. M., Abraham D. J., Leask A. CCN2 is necessary for the function of mouse embryonic fibroblasts. Exp. Cell Res. 2007;313:952–964. doi: 10.1016/j.yexcr.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Kim H., et al. Ultrafine carbon black particles inhibit human lung fibroblast-mediated collagen gel contraction. Am. J. Respir. Cell Mol. Biol. 2003;28:111–121. doi: 10.1165/rcmb.4796. [DOI] [PubMed] [Google Scholar]
- Leask A., Holmes A., Black C. M., Abraham D. J. Connective tissue growth factor gene regulation Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J. Biol. Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Leask A., Abraham D. J. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Leask A. Scar wars: is TGFbeta the phantom menace in scleroderma? Arthritis. Res. Ther. 2006;8:213. doi: 10.1186/ar1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Abraham D. J. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N. L., Frazier-Jessen M., Wahl S. M. TGF-beta: a balancing act. Int. Rev. Immunol. 1998;16(5–6):553–580. doi: 10.3109/08830189809043009. [DOI] [PubMed] [Google Scholar]
- Mimura Y., Ihn H., Jinnin M., Asano Y., Yamane K., Tamaki K. Constitutive phosphorylation of focal adhesion kinase is involved in the myofibroblast differentiation of scleroderma fibroblasts. J. Invest. Dermatol. 2005;124:886–892. doi: 10.1111/j.0022-202X.2005.23701.x. [DOI] [PubMed] [Google Scholar]
- Mori T., Kawara S., Shinozaki M., Hayashi N., Kakinuma T., Igarashi A., Takigawa M., Nakanishi T., Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: a mouse fibrosis model. J. Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Parsons J. T. Focal adhesion kinase: the first ten years. J. Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl. Acad. Sci. USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar E. P., Rozengurt E. Src family kinases are required for integrin-mediated but not for G protein-coupled receptor stimulation of focal adhesion kinase autophosphorylation at Tyr-397. J. Biol. Chem. 2001;276:17788–17795. doi: 10.1074/jbc.M100984200. [DOI] [PubMed] [Google Scholar]
- Serini G., Bochaton-Piallat M. L., Ropraz P., Geinoz A., Borsi L., Zardi L., Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J. Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Shephard P., Hinz B., Smola-Hess S., Meister J. J., Krieg T., Smola H. Dissecting the roles of endothelin, TGF-beta and GM-CSF on myofibroblast differentiation by keratinocytes. Thromb. Haemost. 2004;92:262–274. doi: 10.1160/TH03-11-0669. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X., et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol. Biol. Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi-Wen X., et al. Constitutive ALK5-independent c-Jun N-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: evidence of an autocrine endothelin loop operating through the endothelin A and B receptors. Mol. Cell. Biol. 2006a;26:5518–5527. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi-wen X., et al. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J. Biol. Chem. 2006b;281:10715–10726. doi: 10.1074/jbc.M511343200. [DOI] [PubMed] [Google Scholar]
- Stratton R., Rajkumar V., Ponticos M., Nichols B., Shiwen X., Black C. M., Abraham D. J., Leask A. Prostacyclin derivatives prevent the fibrotic response to TGF-beta by inhibiting the Ras/MEK/ERK pathway. FASEB J. 2002;16:1949–1951. doi: 10.1096/fj.02-0204fje. [DOI] [PubMed] [Google Scholar]
- Thannickal V. J., Lee D. Y., White E. S., Cui Z., Larios J. M., Chacon R., Horowitz J. C., Day R. M., Thomas P. E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- Ventura J. J., Kennedy N. J., Flavell R. A., Davis R. J. JNK regulates autocrine expression of TGF-beta1. Mol. Cell. 2004;15:269–278. doi: 10.1016/j.molcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Verrecchia F., Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J. Invest. Dermatol. 2002;118:211–215. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- Verrecchia F., Vindevoghel L., Lechleider R. J., Uitto J., Roberts A. B., Mauviel A. Smad3/AP-1 interactions control transcriptional responses to TGF-beta in a promoter-specific manner. Oncogene. 2001;20:3332–3340. doi: 10.1038/sj.onc.1204448. [DOI] [PubMed] [Google Scholar]
- Verrecchia F., Mauviel A., Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun. Rev. 2006;5:563–569. doi: 10.1016/j.autrev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Xia Y., Wu Z., Su B., Murray B., Karin M. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 1998;12:3369–3381. doi: 10.1101/gad.12.21.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawel L., Dai J. L., Buckhaults P., Zhou S., Kinzler K. W., Vogelstein B., Kern S. E. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol. Cell. 1998;1:611–617. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Feng X. H., Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394:909–913. doi: 10.1038/29814. [DOI] [PubMed] [Google Scholar]