Abstract
In this study, we investigated the molecular mechanisms underlying the ATP analogue adenosine-5′-O-(3-thio)triphosphate–induced nucleocytoplasmic shuttling of the mRNA stabilizing factor HuR in human (h) mesangial cells (MC). Using synthetic protein kinase C (PKC) inhibitors and small interfering RNA approaches, we demonstrated that knockdown of PKCα efficiently blocked the ATP-dependent nuclear HuR export to the cytoplasm. The functional importance of PKCα in HuR shuttling is highlighted by the high cytosolic HuR content detected in hMC stably overexpressing PKCα compared with mock-transfected cells. The ATP-induced recruitment of HuR to the cytoplasm is preceded by a direct interaction of PKCα with nuclear HuR and accompanied by increased Ser phosphorylation as demonstrated by coimmunoprecipitation experiments. Mapping of putative PKC target sites identified serines 158 and 221 as being indispensable for HuR phosphorylation by PKCα. RNA pull-down assay and RNA electrophoretic mobility shift assay demonstrated that the HuR shuttling by ATP is accompanied by an increased HuR binding to cyclooxygenase (COX)-2 mRNA. Physiologically, the ATP-dependent increase in RNA binding is linked with an augmentation in COX-2 mRNA stability and subsequent increase in prostaglandin E2 synthesis. Regulation of HuR via PKCα-dependent phosphorylation emphasizes the importance of posttranslational modification for stimulus-dependent HuR shuttling.
INTRODUCTION
The modulation of mRNA turnover by the involvement of adenosine/uridine-rich elements (AREs) reflects an important regulatory pathway of posttranscriptional control of gene expression under different physiological and pathological conditions, including cell growth and inflammation as well as cell senescence (for review, see Ross, 1996; Misquitta et al., 2001; Hollams et al., 2002). A tight control of mRNA decay allows rapid alterations in the level of a given mRNA and relevant proteins derived thereof, and it is one prerequisite for a rapid response to changing environmental conditions. Thus, a variety of inducible genes, including proto-oncogenes, transcription factors, cell cycle-regulating proteins, and cytokines, have been demonstrated to be regulated via a modulation of mRNA turnover, and AREs located in the 3′-untranslated regions (UTRs) of these genes comprise specific cis-regulating elements that target mRNAs for rapid degradation (for review, see Chen and Shyu, 1995; Ross, 1996; Fan and Steitz, 1998b; Peng et al., 1998; Fan et al., 2002). Among different proteins specifically binding to AREs, members of the embryonic lethal abnormal vision (ELAV) protein family, especially the ELAV-like protein HuR (HuA), represents one of the best-studied RNA-binding proteins. Unlike the other members of the ELAV family (HuB, HuC, and HuD), which are exclusively found in neuronal tissue, HuR is ubiquitously expressed (Good, 1995; Ma et al., 1996). Functionally, an overexpression and subsequent RNA binding of HuR have been demonstrated to result in a marked stabilization of ARE-containing mRNAs in vivo (Fan and Steitz, 1998a,b). Interestingly, ELAV proteins are abundantly localized in the nucleus, but they undergo a stimulus-dependent shuttling between the nucleus and the cytoplasm (Dreyfuss et al., 2002).
Structurally, the shuttling of HuR is related to a specific HNS shuttling sequence located in the hinge region of the HuR protein (Fan and Steitz, 1998a). Furthermore, the nuclear export of ELAV proteins has been shown to be a target of different signaling pathways, including different members of the mitogen-activated protein kinase (MAPK) family (Winzen et al., 1999; Ming et al., 2001), the AMP-activated kinase family (AMPK) (Wang et al., 2002, 2004), and members of the protein kinase C (PKC) family (Pascale et al., 2005). Using confocal microscopy, we previously demonstrated a nucleocytoplasmic shuttling of HuR after treatment of rat (r)MC with a stable ATP analogue adenosine-5′-O-(3-thio)triphosphate (ATPγS), thereby leading to an increased mRNA stability of matrix metalloproteinase-9 (Huwiler et al., 2003). In rMC, ATP via binding to G protein-coupled P2Y2 receptors is involved in the regulation of a variety of pathophysiological key functions, including cell proliferation, inflammation, and apoptosis (Huwiler and Pfeilschifter, 1994; Pfeilschifter and Huwiler, 1996; Schulze-Lohoff et al., 1998; Huwiler et al., 2002). Because posttranslational modification of HuR by phosphorylation represents a critical feature for HuR shuttling, we hypothesized that the ATP-dependent effects on HuR translocation may be caused by phosphorylation. Computer analysis of human HuR reveals a multitude of putative protein modification sites, including multiple sites of myristoylation, glycosylation, and, importantly, several phosphorylation sites for PKC. By contrast, no typical phosphorylation sites for MAPKs can be found within the protein sequence of HuR. Therefore, it is tempting to speculate that in particular members of the PKC family may play an essential role in the posttranscriptional regulation of HuR.
The human cyclooxygenase (COX)-2 constitutes an important inflammatory mediator that is regulated by the mRNA-stabilizing factor HuR (Dixon et al., 2001). Posttranscriptional regulation is critical in mediating the increased COX-2 levels upon stimulation with proinflammatory stimuli such as interleukin (IL)-1β or lipopolysaccharide, and, interestingly, also by prostaglandin E2 (PGE2) itself (Faour et al., 2001). In the kidney, the COX-2–dependent increase in prostaglandin production is important for the regulation of renal microcirculation. By using an hMC model, we reveal a new mechanism by which ATP activates nucleocytoplasmic HuR shuttling in a PKCα-dependent manner coincident with an increase in COX-2 mRNA stability, implying a further facet of PKC-triggered regulation of gene expression. Furthermore, to the best of our knowledge, we report for the first time that the ubiquitous ELAV protein HuR is a direct target of PKC phosphorylation that is important for its stimulus-dependent export from the nucleus to the cytoplasm.
MATERIALS AND METHODS
Reagents
Human recombinant IL-1β was acquired from Cell Concept (Umkirch, Germany), and human recombinant tumor necrosis factor (TNF)-α was from Knoll (Ludwigshafen, Germany). ATPγS, bryostatin, G-418 (Geneticin), phorbol 12-myristate 13-acetate (PMA), and Ponceau red were purchased from Sigma Chemie (Deisenhofen, Germany). Actinomycin D (Act D) (from Streptomyces species) was purchased from Alexis Biochemicals (Laeufelfingen, Switzerland). The mitogen-activated protein kinase kinase (MEK) inhibitor U0126 and the kinase inhibitors SB203580, PD98059, and Gö6976 were received from Merck Biosciences (Schwalbach, Germany). CGP41251 was a kind gift from Novartis Pharma (Basel, Switzerland). Ribonucleotides and modifying enzymes were purchased from Invitrogen (Karlsruhe, Germany). Protein phosphatase 1 was obtained from Cell Signaling (Frankfurt am Main, Germany). Antibodies specifically raised against HuR; β-actin; COX-2; histone deacetylase 4 (HDAC4); p65 (nuclear factor [NF]-κB); anti-goat, anti-rabbit, and anti-mouse horseradish peroxidase-linked immunoglobulins (IgGs); and Hyperfilm were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). A secondary Alexa Fluoro 488-coupled antibody was from Molecular Probes (Karlsruhe, Germany). Human recombinant PKCα, -δ, -ε, and -ζ were obtained from BIOMOL (Hamburg, Germany). Antibodies against PKCα, phospho-PKCα, PKCδ, and the phospho-(Ser)-PKC-substrate antibody were purchased from Cell Signaling. [32P]dATP (specific activity >3000 Ci/mol), [α-32P]dCTP (specific activity 3000 Ci/mmol), protein G-Sepharose 4B, and enhanced chemiluminescence (ECL) system were purchased from Amersham Biosciences Europe (Freiburg, Germany). The transfection agents Oligofectamine and Lipofectamine 2000 were from Invitrogen. All cell culture media and supplements were purchased from Invitrogen.
Cell Culture
Human primary mesangial cells were isolated from collagenase IV-treated human glomeruli and cultivated as described previously (Radeke et al., 1990). Cells were grown in Roswell Park Memorial Institute 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 5 ng/ml insulin, 100 U/ml penicillin, and 100 μg/ml streptomycin. Serum-free preincubations were performed in Dulbecco's modified Eagle's medium supplemented with 0.1 mg of fatty acid-free bovine serum albumin per milliliter. For experiments, hMC between passages 4 and 10 were used.
Cell Transfections
Gene silencing was performed using small interfering RNAs (siRNAs) for human PKCα (sc-36243), PKCδ (sc-36253), and HuR (sc-35619) (all from Santa Cruz Biotechnology). Transfection of subconfluent hMC was performed by using the Oligofectamine reagent following the manufacturer's instructions (Invitrogen).
Generation of PKCα-overexpressing Mesangial Cell Lines
For generation of an hMC line (hMC-PKCα) stably overexpressing PKCα, hMC were transfected with a pEGFP-PKCα construct described previously (Aschrafi et al., 2003a) by using the Lipofectamine reagent. Stable PKCα-overexpressing cell lines were obtained by selection using 0.6 mg/ml G-418 for 8 wk. Subsequently, clones were isolated, subcultured, and tested for PKCα overexpression by immunoblot. hMC control cell lines (hMC-C) were generated by stable transfection of the pEGFP-C2 vector (Clontech, Palo Alto, CA).
Expression and Purification of Recombinant His-HuR Plasmids
The plasmid pQE-30-His-HuR was generated by subcloning the plasmid pGEX-HuR, which encompasses a cDNA encoding residues 2-326 of human HuR (Ma et al., 1996) into the prokaryotic expression plasmid pQE30 (QIAGEN, Hilden, Germany) by using internal primers. The plasmid pGEX-HuR was a gift from Henry Furneaux (Memorial Sloan Kettering Cancer Center, New York, NY). His-tagged HuR protein was purified by using the QIAexpress kit from QIAGEN following the instructions of the manufacturer. The plasmids pQE-His-HuRΔ1, -Δ2, -Δ3, and -Δ4, each containing a single serine-to-alanine substitution at different positions, were generated by changing a single base (T to G, underlined) by using the (sense) primer pQE-His-HuRΔ1, 5′-TTTGACAAACGGGCGGAGGCAGAAGAGGC-3′, corresponding to a region from nucleotides 459 to 488 of the human HuR cDNA (GenBank accession no. NM_001419); pQE-His-HuRΔ2, 5′-GAGATTCAGGTTCGCCCCCATGGGCGTCG-3′, corresponding to nucleotides 648–676; pQE-His-HuRΔ3, 5′-AAATCTTACAGGTTGCCTTCAAAACCAAC-3′, corresponding to a region from nucleotides 938–966; and pQE-His-HuRΔ4: 5′-AAAACCAACAAGGCCCACAAATAACTCGC-3′, corresponding to nucleotides 957–986. For generation of pQE-30-HuRΔ1/Δ2 bearing two serine-to-alanine substitutions, pQE-His-HuRΔ1 was used as a template and by using the (sense) primer pQE-His-HuRΔ2, corresponding to nucleotides 648–676. All mutants were generated by use of the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
In Vitro Phosphorylation Assay
Phosphorylation of HuR by human recombinant PKCs was tested by an in vitro kinase assay by using HuR as a substrate; the assay was performed as described previously (Geiges et al., 1997). Briefly, 5 μg of recombinant PKC was incubated in PKC assay buffer containing 20 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 10 μM Na2ATP, 25 μg/ml phosphatidylserine, 2.5 μg/ml diolein, 1 μl of [γ-32P]dATP (3000 Ci/mmol), 2 μg of recombinant HuR, and 100 μM CaCl2 in the presence or absence of 100 μM EGTA. Reactions were incubated at 30°C for 20 min, and then directly subjected to polyacrylamide gel electrophoresis (PAGE). After fixing, the gels were vacuum-dried, and radioactive signals were quantified by using a BAS 1500 automated detector system from Fujifilm (Raytest, Straubenhardt, Germany).
Western Blot Analysis
Cytoplasmic and nuclear lysates from cells were prepared by a quick extraction method as described previously (Eberhardt et al., 2002). Western blot analysis was performed using standard procedures as described previously (Eberhardt et al., 2002). After overnight blocking in 2% bovine serum albumin in Tris-buffered saline containing 0.05% Tween, Western blots were probed with the primary antibody for 1 h at room temperature. After incubation with a horseradish peroxidase-conjugated secondary antibody, signals were detected with an ECL system.
Immunoprecipitation of HuR
Nuclear extracts (400 μg) were cleared with protein G beads for 1 h at 4°C before 2 μg of monoclonal anti-HuR antibody, or, alternatively, the same amount of mouse IgG (both diluted in lysis buffer containing 5% fetal calf serum), and extracts were incubated overnight at 4°C. Subsequently, protein G-Sepharose CL-4B beads were added and incubated for another 3 h. After centrifugation for 5 min at 3000 × g, immunocomplexes were successively washed with low salt buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.2% Triton X-100, 2 mM EDTA, 2 mM EGTA, and 0.1% SDS) and high salt buffer (50 mM Tris-HCl, pH 7.5, 500 mM NaCl, 0.2% Triton X-100, 2 mM EDTA, 2 mM EGTA, and 0.1% SDS). After three washing steps, beads were subjected to SDS-PAGE, and coimmunoprecipitated proteins were analyzed by Western blotting.
For detection of PKC-derived phosphorylation, complexes that were immunoprecipitated by the HuR antibody were subsequently immunoblotted with an anti-phospho-(Ser)-PKC-substrate antibody. Phosphate groups from phosphorylated serine residues of nuclear proteins were removed by incubating 500 μg of nuclear extract with 1 U of recombinant protein phosphatase PPT1 for 30 min at 30°C before the immunoprecipitation procedure.
Immunoprecipitation Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
After stimulation, cells were lysed in a buffer containing 10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), 0.1% Nonidet P-40, 50 mM NaF, 10 mM Na3VO4, 10 mM sodium pyrophosphate, 50 mM disodium glycerol phosphate, and 100 U/ml RNasin. Subsequently, cell lysates were immunoprecipitated with 2 μg of either a monoclonal anti-HuR antibody, with an anti-p65 (NF-κB) antibody, or, alternatively, with the same amount of mouse IgG overnight at 4°C. To normalize for equal input of RNA before subsequent purification steps, the same amount of extract was subjected to total RNA isolation by use of the Tri-reagent (Sigma Chemie). Subsequently, protein G-Sepharose CL-4B beads were added and incubated for another 3 h. After centrifugation for 5 min at 3000 × g, beads were successively washed with low and high salt buffer before total RNA was extracted by the Tri-reagent.
RNA Electromobility Shift Assay (EMSA)
RNA gel shift assays for assessment of RNA binding of HuR were accomplished as described previously (Akool et al., 2003). Briefly, a single-stranded RNA oligonucleotide, radioactively labeled by T4 polynucleotide kinase (30 kcpm/reaction), was incubated with 6 μg of cytoplasmic extract and incubated at room temperature for 15 min in a buffer containing 10 mM HEPES, pH 7.6, 3 mM MgCl2, 40 mM KCl, 2 mM DTT, 5% glycerol, and 0.5% Nonidet P-40. To reduce nonspecific binding total yeast RNA (200 ng/ml final concentration) was added. The total volume of each reaction was 10 μl. RNA–protein complexes were separated in 6% nondenaturating polyacrylamide gels and run in Tris borate-EDTA.
The sequence of a RNA oligonucleotide was according to the ARE-III site within the 3′-UTR of the human COX-2 gene (Sengupta et al., 2003), and it is referred to as COX-2-ARE-wt: 5′-GCAUGCUGUUCCUUUUCUUUUCU-3′. COX-2-ARE-mut, which bears six point mutations in the ARE, was used for competition assays (mutated bases are underlined): 5′-GCAUGCUGUUCCUCGCCCGCUCU-3′. Competition experiments were performed by preincubating the binding reaction for 30 min with different dilutions (1:100; 1:300, and 1:1000) of a RNA oligonucleotide stock solution.
qRT-PCR
One-step RT-PCR was performed using a Taqman (ABI 7000) from PerkinElmer-Cetus (Waltham, MA). The mRNA levels for COX-2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were determined by using a “hot start” real-time PCR procedure with Quanti-Tec SYBR Green (QIAGEN). The following oligonucleotides were used as Taqman hybridization probes: COX-2 forward, 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′; COX-2 reverse, 5′-AGATCATCTCTGCCTGAGTATCTT-3′; GAPDH forward, 5′-CACCATCTTCCAGGAGCGAG-3′; and GAPDH reverse, 5′-GCAGGAGGCATTGCTGAT-3′. Calculation of relative COX-2 mRNA levels was done by using the 2 [-ΔΔC(T)] method (Livak and Schmittgen, 2001). According to this method, the C(T) values of COX-2 mRNA level were normalized to the C(T) values of GAPDH mRNA in the same sample.
Determination of PGE2 Level in Conditioned Media
Levels of PGE2 in cell culture supernatants were determined by the Correlate-EIA Prostaglandin E2 enzyme-linked immunosorbent assay (ELISA) kit (Assay Designs, Ann Arbor, MI). hMC were incubated in DMEM without fetal calf serum and stimulated with or without the different agents for 24 h before 100 μl of conditioned media was directly transferred into the microtest strip wells of the ELISA plate. Further procedures were performed following the manufacturer's instructions. The absorbance at 405 nm was measured in a microtest plate spectrophotometer, and PGE2 levels were determined with a calibration curve by using PGE2 as a standard.
Indirect Immunofluorescence Microscopy
The monitoring of nucleocytoplasmic shuttling of HuR by indirect immunofluorescence was performed as described previously (Huwiler et al., 2002). Stained cells were monitored using confocal microscopy (MicroRadiation; Bio-Rad, Hertfordshire, United Kingdom).
Statistical Analysis
Results are expressed as means ± SD. The data are presented as x-fold induction compared with untreated vehicle (*) or compared with stimulated values (#). Statistical analysis was performed using Student's t test and analysis of variance (ANOVA) for significance. p values <0.05 (* and #), <0.01 (** and ##), and <0.005 (***) were considered significant.
RESULTS
The Nucleocytoplasmic Shuttling of HuR by ATP Critically Depends on PKC
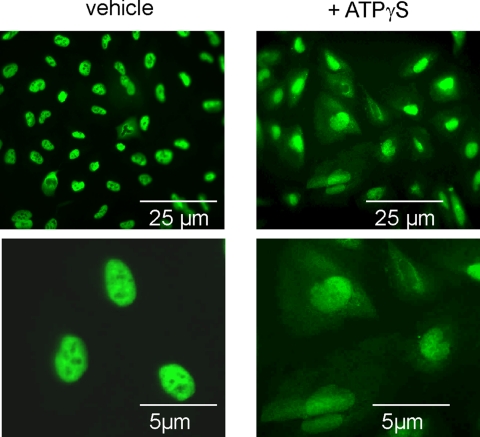
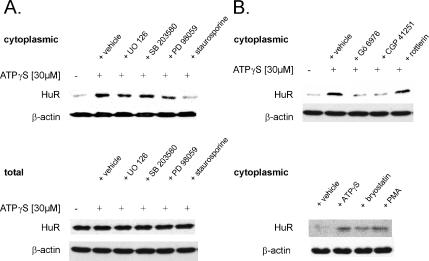
The intracellular trafficking of the ubiquitously expressed mRNA stabilizing factor HuR represents an important mode of regulation of ARE-containing mRNAs via involvement of different kinases, including the phosphatidylinositol-3 (PI-3)-kinase (Ming et al., 2001), the p38 MAPK (Winzen et al., 1999; Ming et al., 2001), and the AMPK (Wang et al., 2002, 2004). To investigate whether in addition to these key signaling pathways PKC may play a role in the nucleocytoplasmic shuttling of HuR in hMC, we tested the effects of the physiological PKC activator ATP, which we previously have identified as a modulator of HuR-dependent mRNA stability in glomerular rMC (Huwiler et al., 2003). By indirect immunofluorescence, we found that under basal conditions HuR is exclusively present within the nucleus, but upon stimulation with the stable ATP analogue ATPγS at 30 μM, it partially shuttles to the cytoplasm, which is paralleled by a diminuation in nuclear staining (Figure 1). The effects of ATP were most obvious after 4 h of stimulation and concomitant with an increase in the cytoplasmic HuR protein content as shown by Western blot analysis (Figure 2A). Furthermore, the ATP-imposed increase in cytoplasmic HuR did not result from overall increased HuR expression, because the total cellular HuR content remained unchanged (Figure 2A, bottom). To prove the involvement of PKC in the ATP-dependent HuR shuttling, we tested the modulatory effects of pharmacological inhibitors targeting different signaling pathways: U0126 (20 μM), an inhibitor of MEK; SB203580 (10 μM), a specific inhibitor of the p38 pathway; PD98059 (30 μM), an inhibitor of the p42/p44 MAPK module; and staurosporine (100 nM), a broad-spectrum PKC inhibitor. Whereas SB 203580, U0126, and PD98059 had no or only slight effects on cytoplasmic HuR levels, staurosporine caused a strong reduction of the ATP-evoked cytosolic HuR accumulation (Figure 2A). Use of 20 nM Gö6976 and 100 nM CGP41251, which both display a high selectivity toward Ca2+-dependent classical PKCs isozymes, similar to staurosporine, strongly abrogated the ATP-induced increase in cytoplasmic HuR levels (Figure 2B, top). By contrast, 10 μM rottlerin, an inhibitor with a high selectivity for the novel and Ca2+-independent PKCδ and PKCθ isoenzymes, caused only weak alterations in the cytosolic HuR accumulation (Figure 2B, top).
Figure 1.
The stable ATP analogue ATPγS induces a nuclear export of HuR in hMC (left). Indirect immunofluorescence was applied to visualize changes in the subcellular localization of HuR by ATP (note the different magnification in the top and bottom panels). Quiescent hMC were stimulated for 4 h with either vehicle or with 30 μM ATPγS as indicated before cells were fixed and stained with an anti-HuR antibody and subsequently with anti-mouse-Alexa 488 used as a secondary antibody. Data are representative of two independent experiments giving similar results.
Figure 2.
PKC inhibition prevents the cytoplasmic accumulation of HuR by ATP. (A) Representative Western blot analysis showing stimulus-dependent changes in the cytoplasmic (top) and total HuR levels (bottom) in hMC. Cells were serum-starved for 16 h and subsequently remained untreated (−) or were treated for additional 4 h with 30 μM ATPγS in the absence (vehicle) or presence of either 20 μM UO126, 10 μM SB203580, 30 μM PD98059, or 100 nM staurosporine as indicated. All inhibitors were preincubated for 30 min before the addition of ATPγS. Protein lysates (50 μg) from cytoplasmic (top) fractions or whole cell extracts (bottom) were subjected to SDS-PAGE and immunoblotted with an anti-HuR–specific antibody. To correct for variations in the protein loading, the blots were stripped and reincubated with an anti-β-actin antibody. (B) Effects of different PKC inhibitors (top) or PKC activators (bottom) on ATP-induced HuR accumulation in cytoplasmic fractions. hMC were serum starved for 16 h and subsequently remained untreated (−), or they were treated for additional 4 h with 30 μM ATPγS in the absence (+vehicle) or presence of either 20 nM Gö6976, 100 nM CGP 41251, or 10 μM rottlerin. hMC were treated for 4 h with either vehicle, 30 μM ATPγS, 50 nM bryostatin, or 100 nM PMA as indicated, and HuR cytoplasmic levels were assessed by Western blot analysis. The Western blots shown are representative of two independent experiments giving similar results.
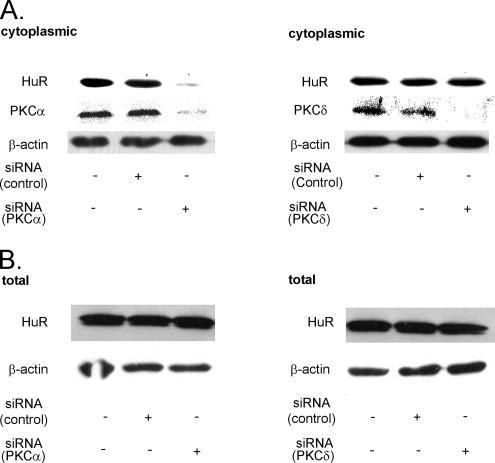
Furthermore, the ATP-induced HuR shuttling was mimicked by the direct PKC activators phorbol ester PMA (100 nM) and bryostatin (50 nM) (Figure 2B, bottom). To further support the results with pharmacological inhibitors, we adopted a model of PKCα knockdown by silencing siRNA technique. Transient transfection with PKCα-siRNA resulted in a strong reduction in total PKCα levels concomitant with a marked reduction in the ATP-caused HuR accumulation within the cytoplasm, whereas transfection of a none-gene–related control siRNA did not affect the HuR recruitment to the cytosol (Figure 3A, left). By contrast, silencing of PKCδ, a PKC isozyme that similar to PKCα shows a high abundance in renal MC (Aschrafi et al., 2003b), had no effect on the ATP-induced increase in cytoplasmic HuR levels, indicating that the ATP-induced recruitment of HuR to the cytoplasm critically depends on the PKCα isoform (Figure 3A, right). By contrast, the total HuR level was not affected by attenuation of either PKC isoform (Figure 3B), indicating that the reduction in the cytosolic HuR levels are not due to an overall decrease in the total HuR protein content.
Figure 3.
Silencing of PKCα, but not of PKCδ, impairs the ATP-induced increase in cytosolic HuR levels (A) but does not affect the total HuR content (B). MC were transfected with either vehicle (−) or siRNA duplexes of human PKCα (left), or, alternatively, of human PKCδ (right), or control siRNA duplexes as described in Materials and Methods. After transfection, serum-starved MC were stimulated with 30 μM ATPγS for 4 h before being harvested for cytoplasmic (A), or total (B) cell fractions, respectively. Protein lysates (50 μg) from both fractions were subjected to SDS-PAGE and successively immunoblotted with the indicated antibodies. To correct for variations in the protein loading, blots were stripped and reprobed with an anti-β-actin antibody. The Western blots shown are representative of three independent experiments giving similar results.
ATP Induces a Physical Interaction of PKCα with HuR
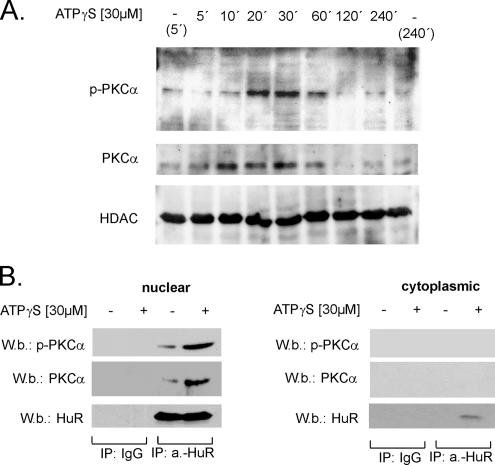
To further test whether PKC-dependent effects on recruitment of HuR to the cytoplasm were preceded by an ATP-triggered PKCα translocation to the nucleus, we performed Western blot analysis with nuclear extracts by using phospho-specific and phosphorylation-independent anti-PKCα antibodies, respectively. Time course experiments revealed that ATP promoted a rapid and transient appearance of phosphorylated PKCα within nuclear fractions that was maximal after 20 min of stimulation and thereafter declined back to basal levels (Figure 4A). Concomitantly, we observed a transient increase in the total PKCα level in the nuclear extracts, indicating that the ATP-induced increase in nuclear PKCα phosphorylation is primarily due to a stimulus-dependent translocation of an already phosphorylated form of PKCα. In contrast, the level of HDAC4 that was determined for monitoring of equal nuclear protein loading was not changed by ATP treatment (Figure 4A). Furthermore, we tested whether ATP may evoke a direct physical interaction of PKCα with nuclear HuR by coimmunoprecipitation (IP). For IP, a monoclonal anti-HuR antibody, or alternatively, as a negative control, mouse IgG was used and followed by Western blotting with antibodies against total PKCα and phospho-specific PKCα, respectively. ATP induced a substantial increase in the levels of PKC and phospho-PKC coimmunoprecipitated by an anti-HuR antibody, whereas the level of immunoprecipitated HuR remained unchanged (Figure 4B, left). By contrast, no immunoprecipitated PKCα, either in its phosphorylated or in its unphosphorylated state, could be detected when instead of nuclear extracts, an equal protein amount of the corresponding cytoplasmic fraction derived from the same experiment was used as input material in the immunoprecipitation experiment (Figure 4B, right). Collectively, these data demonstrate that ATPγS initiates a rapid translocation of PKCα to the nucleus that is accompanied by an increased interaction with nuclear HuR.
Figure 4.
Nuclear translocation of PKCα by ATPγS is accompanied with an increased binding of PKCα to nuclear HuR. (A) Time course of PKCα translocation to the nucleus by ATPγS. Quiescent hMC were treated with either vehicle (−) or with 30 μM ATPγS and lysed after the indicated times. Nuclear extracts (50 μg) were subjected to SDS-PAGE and successively immunoblotted with a phosphorylation-independent (PKCα) and a phospho-specific (p-PKCα) PKCα antibody. To ascertain equal protein contents within the nuclear fractions, the blots were stripped and reprobed with an HDAC4-specific antibody. The Western blots shown are representative of three independent experiments giving similar results. (B) IP-phospho-PKCα (P-PKCα) and PKCα (PKCα) levels after an overnight incubation with 2 μg of either anti-HuR antibody (a.-HuR) or an irrelevant IgG isotype antibody (IgG) used as a negative control. For IP, a total protein amount (500 μg) of either nuclear (left) or cytoplasmic extract (right) and derived from either untreated (−) or ATP-treated hMC (+) was taken, and immunocomplexes were separated by Sepharose G as described in Materials and Methods. Equal amounts of immunoprecipitated HuR were ascertained by stripping the blot and reincubating with the anti-HuR antibody also used for IP. The data shown are representative of three independent experiments giving similar results.
Overexpression of PKCα Is Accompanied by Increased Cytosolic HuR Levels
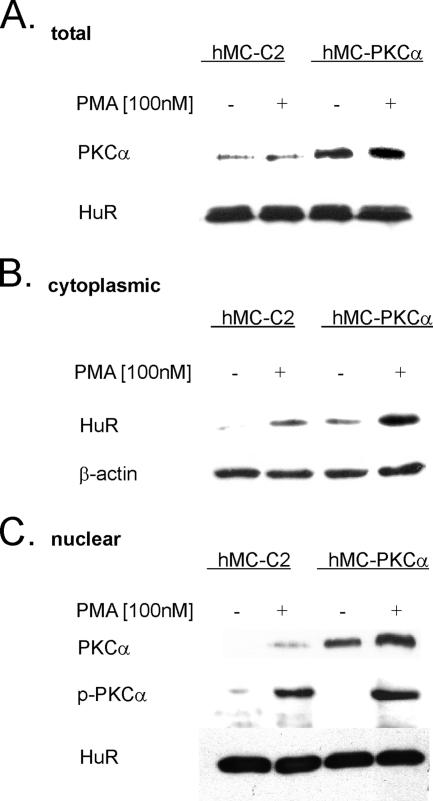
To further substantiate PKCα involvement in the nucleocytoplasmic HuR shuttling, we generated a human mesangial cell line that stably overexpresses PKCα (hMC-PKCα), resulting in an overall increase in the total cellular PKCα content (Figure 5A). Similar to the situation observed in untransfected hMC, cytoplasmic fractions from untreated mock-transfected control cells (hMC-C2) contained almost no detectable HuR, whereas a short-term stimulation (30 min) with 100 nM PMA caused an increase in cytosolic HuR levels (Figure 5B). In contrast to the mock-transfected cells, hMC-PKCα cells, even under unstimulated conditions contained cytosolic HuR, and, importantly, this amount of cytosolic HuR is strongly increased upon treatment with PMA (Figure 5B). Correspondingly, cells stably overexpressing PKCα contained much higher PKCα levels in the nucleus under basal and phorbol ester-stimulated conditions than mock-transfected cells (Figure 5C). Interestingly, overexpression of PKCα was not accompanied by an equivalent raise in PKCα phosphorylation, indicating that in hMC, an overexpression of PKCα, independent of its phosphorylation status, can induce a nuclear cytoplasmic shuttling of HuR.
Figure 5.
PKCα-overexpressing hMC induce HuR shuttling. Mock-transfected MC (hMC-C2) or cells stably overexpressing PKCα (hMC-PKCα) were serum starved for 16 h and subsequently treated for 30 min with either vehicle (−) or with 100 nM PMA (+). Subsequently, cells were harvested and subjected to isolation of either total (A), cytoplasmic (B), or nuclear extracts (C). Fifty micrograms of the respective fraction was subjected to SDS-PAGE and immunoblotted with the indicated antibodies. To correct for equal protein loading, the blot from the cytoplasm was stripped and reincubated with an anti-β-actin antibody (B), whereas the blot from the nuclear fraction was probed with the anti-HuR antibody (C). The blots shown are representative of three independent experiments with similar results.
In Vitro Phosphorylation of HuR by PKCα
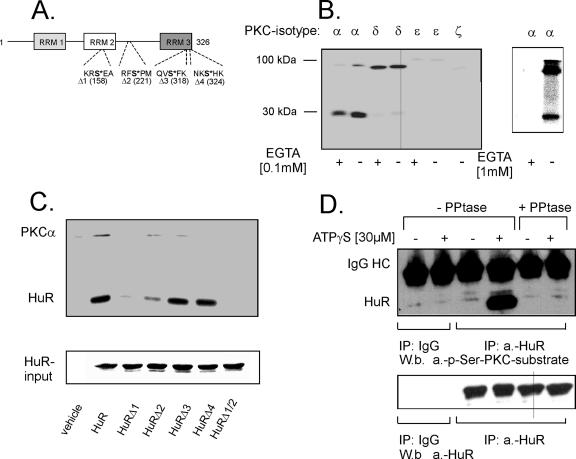
Based on the observed physical interaction of HuR with PKCα, we sought to examine whether HuR is a direct target of PKC. The human amino acid sequence of HuR reveals several putative conserved PKC/Ser phosphorylation sites (Figure 6A). Therefore, we tested whether recombinant PKC isoforms phosphorylate HuR in a cell-free PKC assay. Purified bacterially expressed HuR was subjected to phosphorylation by the different PKC isoenzymes (α, δ, ε, and ζ) expressed in MC (Aschrafi et al., 2003b). Furthermore, to test for a Ca2+ dependence, the kinase assays were performed either in the presence or in the absence of the Ca2+ chelator EGTA. The appearance of a phosphorylated radioactive band at 34 kDa, corresponding to the migration properties of HuR, was detected in the presence of PKCα and with a very weak intensity also with PKCδ and PKCε but not with PKCζ (Figure 6B, left). Consistent with the known dependence of PKCα activity on Ca2+, a moderate reduction in HuR phosphorylation was found by removal of Ca2+ by 0.1 mM EGTA, and a total inhibition in phosphorylation was observed with a high EGTA concentration of 1.0 mM (Figure 6B, right). Furthermore, a second phosphorylated band, migrating at ∼80–90 kDa and corresponding to autophosphorylated PKCs, was observed with all PKC isoforms (Figure 6B, left).
Figure 6.
PKC-mediated in vitro phosphorylation of recombinant HuR. (A) Schematic representation of HuR and the relative positions of putative conserved PKC/serine phosphorylation sites (Arabic numbers indicate the amino acid position). For generation of single HuR point mutations (HuRΔ1, HuRΔ2, HuRΔ3, and HuRΔ4) and of the double mutation HuRΔ1/2, serines (bold; asterisk) at the indicated positions (bracket) were substituted by alanine. RRM, RNA recognition motif. (B) Two micrograms of bacterially produced His-tagged HuR was incubated with 5 μg of the indicated recombinant PKC isoforms in a cell-free phosphorylation assay in the presence of [γ-32P]ATP and with (+) or without (−) of 100 μM EGTA. To further validate Ca2+ dependency of PKCα, phosphorylation of this isoenzyme was additionally tested in the presence of a higher amount of EGTA (1 mM) (Figure 6B, right). Equal protein loading was ascertained by Coomassie blue staining (data not shown). An autoradiograph of one representative gel is shown. Radioactive bands migrating at 78–97 kDa represent autophosphorylated PKCs. The assay shown is representative of two further experiments giving similar results. (C) In vitro PKC phosphorylation in the absence (vehicle) or presence of either wild-type HuR or point mutated HuR by using recombinant PKCα and [γ-32P]ATP in the absence of EGTA. Equal amounts of recombinant HuR were ascertained by Coomassie blue staining (input HuR). (D) Top, HuR phosphorylation of serine is induced in response to ATPγS stimulation. One milligram of nuclear extract derived from untreated (−) or ATPγS-treated hMC (+) was immunoprecipitated with a monoclonal anti-HuR antibody (IP: a.-HuR), or, alternatively with mouse IgG (IP: IgG). HuR phosphorylation was tested by Western blotting by using a p-Ser-PKC-substrate antibody. The antibody specificity was assessed by pretreatment of the immunoprecipitated eluates with protein phosphatase (+PPtase). Bottom, incubation with the anti-HuR antibody (W.b. a.-HuR) demonstrates equal HuR precipitation. The data shown are representative of three independent experiments giving similar results.
Mapping of Putative PKC Phosphorylation Sites by Site-directed Mutagenesis
We next aimed to identify the sites within the HuR protein critically involved in the PKCα-dependent HuR phosphorylation. Although a protein motif scan of the amino acid sequence of HuR revealed no perfect match of a PKC phosphorylation site (characterized by a serine within the following amino acid context: X-X-R/K-X-S-hydrophobic residue-R/K), we found four positions in which the amino acid serine is surrounded by arginine (R) or lysin (K) at either the −2 or the +2 position and by a hydrophobic residue (Hyd) at the +1 position (Figure 6A). To determine which, if any, of these putative PKC phosphorylation sites are critical for HuR phosphorylation by PKCα, we generated four different point mutations in the context of full-length HuR, performing single serine-to-alanine substitutions within the coding region. Mutation in serine 158 (HuR-Δ1) led to a complete inhibition of phosphorylation, and serine 221 (HuRΔ2) yielded a somewhat lesser reduction in phosphorylation, whereas mutations in serine 318 (HuR-Δ3) or serine 324 (HuR-Δ4) did not affect HuR phosphorylation by PKCα (Figure 6C). Similarly to HuR-Δ1, double mutation of serines 158 and 221 (HuRΔ1/Δ2) resulted in a complete loss of in vitro phosphorylation by PKCα, indicating that although serine 158 and serine 221 both are indispensable for full HuR phosphorylation, deletion of serine 158 leads to a stronger impairment of HuR phosphorylation by PKCα (Figure 6C). However, because in the cell-free PKC assay all participating factors are added in an overall excess, an exact determination of stoichiometrical relationships between single sites existing under in vivo situtations is not possible by this approach.
Treatment of hMC with ATP Induces a PKC-specific Serine Phosphorylation of Nuclear HuR
To further test whether the PKC-dependent phosphorylation of HuR is also observed in living cells, we performed coimmunoprecipitations, making use of a phospho-serine PKC substrate antibody (p-Ser-PKC-substrate). This antibody recognizes proteins only when phosphorylated on PKC-specific serine consensus sites described above. To this end, nuclear extracts derived from untreated or ATPγS-treated hMC were immunoprecipitated with a monoclonal anti-HuR antibody, or, alternatively, with mouse IgG used as a negative control. The immunoprecipitates were subsequently probed with the p-Ser-PKC-substrate antibody. Treatment of hMC with ATP for 40 min caused appearance of a strong band coimmunoprecipitated by the HuR antibody and corresponding to the migration properties of HuR, indicating a stimulus-dependent phosphorylation at PKC-specific serines of HuR (Figure 6D, top). However, the serine-phosphorylated PKC-substrate–specific band disappeared when the nuclear extracts, before the IP, were subjected to phosphatase treatment, demonstrating the specificity of the p-Ser-PKC-substrate antibody (Figure 6D, top). By contrast, the amount of immunoprecipitated HuR remained unchanged independently of how cells were treated (Figure 6D, bottom).
ATP Augments HuR Binding to a COX-2-specific ARE
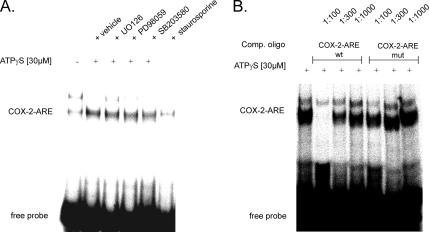
Next, we performed RNA EMSA to test whether stimulation of cells with ATP induces HuR binding to an ARE-containing mRNA. We used an RNA oligonucleotide encompassing an ARE from the human 3′-UTR of COX-2 (COX-2-ARE), which represents a well-known functional binding element for HuR (Sengupta et al., 2003). To this end, the same cytoplasmic extracts used before for the assessment of nuclear cytoplasmic HuR shuttling (Figure 2) were tested for in vitro RNA binding. We observed the constitutive binding of one prominent complex whose binding is strongly induced upon ATP stimulation (Figure 7A). In contrast, the binding of a weaker and slower migrating complex was abrogated by ATPγS (Figure 7A). Coincident with the modulation of HuR shuttling by pharmacological inhibitors, the ATP-induced ARE binding was specifically abrogated when cells had been stimulated in the presence of the PKC inhibitor staurosporine, but it was not affected by any of the other inhibitors tested (Figure 7A). Furthermore, RNA binding of the ATP-induced complex was strongly competed by a nonlabeled wild-type oligonucleotide (COX-2-ARE-wt) (Figure 7B). In contrast, competition with the unlabeled mutant oligonucleotide (COX-2-ARE-mut) was much less effective, and a weak reduction in RNA binding was only observed at the highest concentration of the competing oligo (1:100) (Figure 7B). These data indicate that the ARE motif is necessary for retention of a full competition capacity in ATP-induced RNA binding to the 3′-UTR of COX-2.
Figure 7.
ATP increases the RNA binding of cytoplasmic complexes to a COX-2–specific ARE. Representative EMSA using an ARE-containing oligonucleotide from the 3′-UTR of the human COX-2 mRNA. (A) Cells were treated for 4 h with 30 μM ATPγS in the absence (vehicle) or presence of either 20 μM UO126, 10 μM SB203580, 30 μM PD98059, or 100 nM staurosporine before being lysed for preparation of cytoplasmic extracts. The conditions for RNA binding were as described under Materials and Methods. (B) Competition capacities of wild-type (wt) and mutant (mut) COX-2 AREs. Different molar excess (depicted as different dilutions of an oligonucleotide stock solution) of the indicated competitor oligonucleotide was added 30 min before the addition of the 32P-labeled wild-type oligonucleotide (COX-2-ARE). The sequences of the oligonucleotides are depicted in Materials and Methods. The EMSAs shown are representative of two independent experiments giving similar results.
ATP-Caused Increase in HuR Binding to COX-2 Transcripts Results in an Amplification of COX-2 mRNA Stability
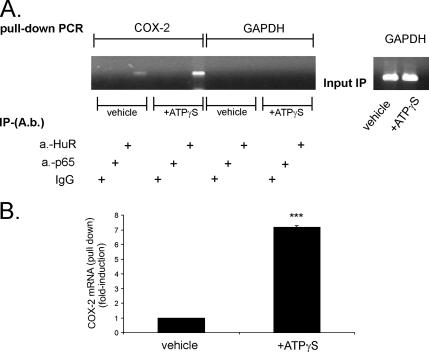
To test whether ATP treatment causes an increase in HuR interaction with COX-2 mRNA in vivo, we made use of a pull-down assay in which HuR was immunoprecipitated in cytoplasmic extracts from hMC. By this approach, COX-2 transcripts, when bound to HuR, are accumulated and subsequently characterized by RT-PCR (Figure 8, top). COX-2 mRNA shows a weak constitutive binding to HuR under unstimulated conditions (1-fold), which is strongly increased upon treatment with ATPγS (8 ± 0.04-fold; mean ± SD; n = 3) (Figure 8B). The difference in COX-2 cDNA levels amplified after the IP with the anti-HuR antibody was not due to an overall difference in the total mRNA levels, because cytosolic extracts used for the IP contained a similar amount of GAPDH mRNA (Figure 8A, right, input IP). Furthermore, no RNA was amplified when instead of the anti-HuR antiserum, an antibody raised against the NF-κB subunit p65 (p65), or, alternatively, serum IgG was used for the IP (Figure 8A). Also, no RNA was amplified when a primer pair specific for GAPDH mRNA, another ARE-containing mRNA, was used for PCR (Nagy and Rigby, 1995) (Figure 8A), which indicates a gene specificity of the observed mechanisms.
Figure 8.
ATP increases the intracellular binding of HuR to COX-2 mRNA but not to the ARE-containing GAPDH mRNA. (A) hMC were treated for 4 h with vehicle or with 30 μM ATPγS before being immunoprecipitated with 2 μg of either anti-HuR (a.-HuR), an anti-NF-κB subunit p65-specific (a.-p65) antibody, or with the same amount of mouse IgG (IgG). RNA bound by HuR was harvested and subjected to qRT-PCR by using either COX-2–specific primers or primers specific for GAPDH. Normalization of similar amount of input RNA added to the immunoprecipitation reactions was done by assessment of GAPDH levels (input IP) (A, right). Top, representative semiquantitative RT-PCR reactions. A graphic summary of a representative triplicate qRT-PCR-experiment (of 2 experiments) is depicted in B. Results are expressed as means ± SD (n = 3) and are presented as -fold induction (p ≤ 0.005) compared with vehicle (***).
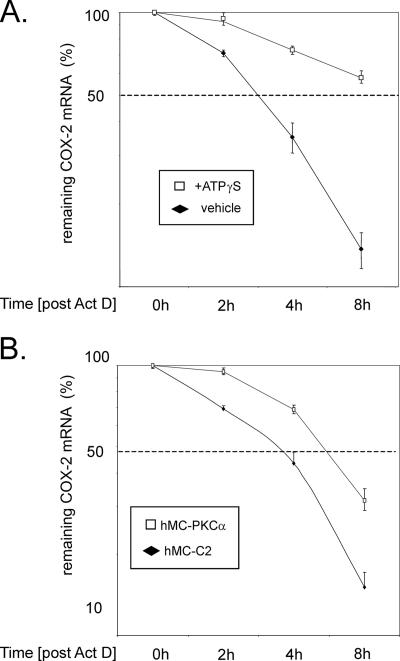
To furthermore address whether the ATP-increased binding of HuR to COX-2 mRNA is functionally linked with an increase in COX-2 mRNA stability, we performed experiments with actinomycin D. MC were stimulated with TNF-α and IL-1β (both at 2 nM) for 16 h to allow a profound induction of COX-2 expression before transcription was blocked by 5 μg/ml actinomycin D (Figure 9A). Subsequently, cells were either directly homogenized (0 h) or left untreated (+vehicle), or, alternatively, they were treated with 30 μM ATPγS before total RNA was isolated after 2, 4, and 8 h, respectively. Expression of COX-2 in comparison with GAPDH was determined by qRT-PCR. The half-life of COX-2 mRNA was substantially increased by ATP from almost 3 h to >8 h (Figure 9A).
Figure 9.
The ATP-triggered increase in cytokine-induced COX-2 mRNA stability can be mimicked by overexpression of PKCα. (A) Serum-starved hMC were stimulated for 16 h with a cytokine mix containing IL-1β and TNF-α (both at 2 nM) and then washed twice before 5 μg/ml actinomycin D (Act D) was added. After a short preincubation of 30 min, cells were additionally treated for the indicated times with vehicle (filled rhombi) or with 30 μm ATPγS (open squares) before being harvested and extracted for total cellular RNA. At the indicated times, COX-2 mRNA levels were quantified by qRT-PCR by using GAPDH as normalization control. (B) The stability of COX-2 mRNA from hMC-PKCα–overexpressing mesangial cells is substantially increased compared with mock-transfected control cells. hMC-PKCα (open squares) and hMC-C2 cells (filled rhombi) were preincubated for 16 h in serum-free culture medium before 5 μg/ml Act D was added (0 h). After the indicated times, cells were extracted for total cellular RNA, and the decay of COX-2 mRNA was analyzed by qRT-PCR by normalizing COX-2 mRNA to GAPDH mRNA. Graphs show means ± SD (n = 3) and depict the percentage of remaining COX-2 mRNA compared with the level of COX-2 mRNA measured at 0 h. The data shown are representative of two independent experiments with similar results.
Overexpression of PKCα Results in an Increase in COX-2 mRNA Stability
Based on the observation that HuR export to the cytosol by ATP is triggered by PKCα and thereby can cause an increase in COX-2 mRNA stability, we tested whether the hMC line hMC-PKCα stably overexpressing PKCα would display an increased half-life in COX-2 mRNA. Therefore, serum-starved control cells (hMC-C2) or hMC-PKCα cells were treated with 5 μg/ml actinomcyin D to block transcription. The decay of constitutive COX-2 mRNA levels was subsequently monitored by qRT-PCR. As shown in Figure 9B, the relative half-life of COX-2 mRNA from PKCα-overexpressing MC differed substantially from the mock-transfected control cells, and it increased from <4 h in control cells (hMC-C2) to almost 6 h in hMC-PKCα cells (Figure 9B). In summary, these data corroborate our suggestion that an increase in cytoplasmic HuR accumulation by PKCα is functionally linked with an increase in COX-2 mRNA stability, and they further support the notion that the ATP-triggered stabilization of COX-2 mRNA is primarily due to a PKCα-triggered increase in the cytosolic HuR accumulation.
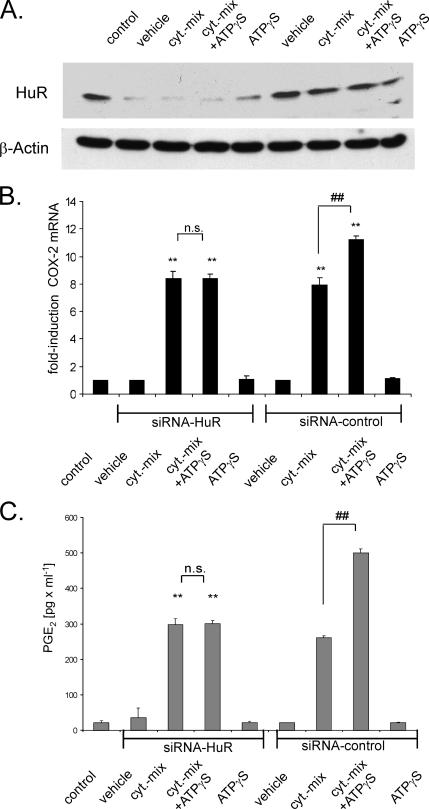
Silencing of HuR Prevents the ATP-dependent Increase in COX-2 Expression
To further prove a requirement for HuR in the ATP-induced posttranscriptional amplification of cytokine-evoked COX-2, we used an siRNA approach to silence HuR expression. After transfection, hMC were either left untreated (vehicle) or stimulated for 24 h with a cytokine mix (TNF-α and IL-1β; 2 nM each) in the absence or presence of 30 μM ATPγS before the steady-state mRNA level of COX-2 was monitored by qRT-PCR (Figure 10B). Assessment of total HuR content by Western blot analysis demonstrated that the level of HuR was strongly diminished in cells transfected with HuR siRNA but that it remained unchanged by the control siRNA (Figure 10A). Interestingly, silencing of HuR exclusively impaired the ATP-triggered amplification in cytokine-induced COX-2 mRNA level without affecting basal or cytokine-evoked COX-2 mRNA levels (Figure 10B). ATP on its own did not cause an increase in COX-2 steady-state mRNA levels independently from whether HuR was attenuated (Figure 10B). This is a discrepancy from the results from pull-down assays, where we found an ATP-induced increase in the coimmunoprecipitated COX-2 mRNA (Figure 8). This apparent contradiction may be explained by the fact that in pull-down assays, the COX-2 mRNA is highly enriched by IP before the PCR amplification; therefore, even slight differences in COX-2 mRNA amounts may become detectable. Consistent with changes in COX-2 mRNA, silencing of HuR strongly impaired the amplification of the cytokine-induced PGE2 release by ATPγS as measured by ELISA (Figure 10C). Again, silencing of HuR did not affect the cytokine-evoked induction in PGE2 synthesis, demonstrating that in hMC HuR is indispensable for the amplification of COX-2 by ATP.
Figure 10.
Silencing of HuR prevents the amplification of cytokine-induced COX-2 expression and PGE2 release by ATP. MC were either left untransfected (control), or they were transfected with either siRNA duplexes of human HuR (siRNA-HuR) or control siRNA duplexes (siRNA-control) as described in Materials and Methods. After transfection, cells were serum starved for 16 h before being treated for a further 24 h with vehicle or with a cytokine mix (Cyt-mix.) containing IL-1β and TNF-α (both at 2 nM) in the presence (+ATPγS) or absence of ATPγS (30 μM) or with ATPγS alone as indicated. The efficiency of silencing RNA on HuR protein levels was monitored by assessment of the total HuR level by Western blot analysis by using an anti-HuR–specific antibody. To correct for variations in protein loading, the blot was stripped and reincubated with an anti-β-actin antibody (A). (B) Changes in the COX-2 mRNA levels were determined by qRT-PCR by normalizing COX-2 mRNA to GAPDH mRNA. The results are means ± SD (n = 3), and they are presented as -fold induction (p ≤ 0.01) compared with nonstimulated controls (**) or to cytokine-stimulated values (##). n.s., not significant. (C) PGE2 levels in cell supernatants derived from hMC treated as indicated and described in detail in A. Data represent means ± SD (n = 3). p ≤ 0.01 compared with control (**) or with cytokine-induced conditions (##).
DISCUSSION
Several studies have highlighted the essential role of the ubiquitously expressed ELAV protein HuR in stabilizing a variety of labile mRNAs bearing AREs within their 3′-UTRs (for review, see Ross, 1996; Hollams et al., 2002). However, less information is available on the signaling pathways triggering HuR function. That HuR shows a predominant nuclear localization but exerts its mRNA-stabilizing function by shuttling between the nucleus and the cytoplasm is an important issue is the identification of signaling pathways regulating the stimulus-dependent nuclear export of HuR. So far, three main signaling cascades modulating nucleocytoplasmic shuttling of HuR have been described involving the p38 MAPK pathway (Winzen et al., 1999; Ming et al., 2001), PI-3-kinase (Ming et al., 2001), and AMPK (Wang et al., 2002, 2004). In contrast, the neuronal ELAV proteins HuB, HuC, and HuD are regulated by PKC, which is functionally important for the regulation of GAP-43 mRNA stability (Pascale et al., 2005). In this study, we demonstrate that PKCα is not only an important trigger of neuronal Hu proteins but also a master switch of nucleocytoplasmic shuttling of the ubiquitous ELAV protein HuR. Using a variety of pharmacological inhibitors, we found that the ATP-dependent HuR shuttling in human MC is strongly impaired by specific PKCα inhibitors. Consistently, silencing of PKCα profoundly impaired the ATP-stimulated HuR translocation to the cytosol, whereas knockdown of PKCδ had no effect on ATP-induced HuR shuttling. This provides evidence that PKCα among the four PKC isoenzymes abundantly expressed in MC (PKCα, -δ, -ε, and -ζ) has a critical role in this process. Physiologically, ATP via binding to the P2Y2 receptor and subsequent phospholipase C activation and Ca2+ mobilization (Pavenstadt et al., 1993) elicit a myriad of signals that besides PKC, include the classical p42/p44 MAPK cascade (Huwiler and Pfeilschifter, 1994), the stress-activated c-Jun NH2-terminal kinase (JNK), and the p38-MAPK cascade (Huwiler et al., 1997), and finally, the PI-3-kinase/protein kinase B cascade (Huwiler et al., 2002). Furthermore, high levels of ATP were shown to inhibit the activity of AMPK, thereby elevating the level of HuR in human colorectal carcinoma cells (Wang et al., 2002). Future experiments need to address the role of AMPK in the ATP-induced HuR shuttling in hMC. By coimmunoprecipitation experiments, we could demonstrate a physical interaction between PKCα and HuR in the nuclear compartment. In this context, it is tempting to speculate that activation of PKCα causes a direct posttranscriptional modification of HuR, thereby facilitating its export to the cytoplasm. So far, methylation of HuR, which is functionally involved in the lipopolysaccharide-induced stabilization of TNF-α mRNA, is the only HuR modification that has been demonstrated (Li et al., 2002). Using a cell-free in vitro kinase assay, we demonstrate for the first time that HuR is a direct substrate of PKCα. This observation corroborates a previous finding from Pascale et al. (2005) demonstrating a PKCα-dependent regulation of neuronal ELAV proteins. Due to the existence of several conserved putative threonine PKC phosphorylation sites and by the use of IPs with a phosphothreonine-specific antibody, they suggested a direct HuR phosphorylation by PKCα on threonine residues. This is in contrast to the PKCα-dependent phosphorylation of HuR at serine 158 and 221, both sites being critical for phosphorylation by PKCα as shown in this study. The functional relevance of a PKCα-dependent HuR phosphorylation at serines in hMC is furthermore highlighted by the strong ATP-dependent induction of phosphorylation on PKC consensus sites of nuclear HuR observed by use of a phospho-Ser-PKC-substrate–specific antibody. Comparison of the amino acid sequence of the nELAV proteins with that of HuR further revealed that the nELAV proteins do not show comparable PKC/Ser sites, indicating a striking heterogeneity in the mode of PKC regulation between neuronal ELAV proteins and the ubiquitous HuR. Concerning the complex mechanisms regulating a bidirectional HuR transport, previous studies could demonstrate that HuR is bound by various associated proteins, including factors such as SETs, pp32, or APRIL, the latter being indispensable for HuR nuclear export (Gallouzi et al., 2001; Moore and Rosbash, 2001). Both APRIL and pp32 interact via their leucine-rich nuclear signals with the nuclear export receptor chromosome maintenance region 1 (CRM1), a member of the importin β family (Fan and Steitz, 1998a). Most intriguingly, leptomycin B, a fungal inhibitor of CRM1-dependent export, also causes a substantial reduction in COX-2 mRNA stability (Jang et al., 2003). Conversely, interactions with transportins via the HNS sequence of the HuR protein are most likely important for the reimport of cytosolic HuR back to the nucleus (Rebane et al., 2004). Whether HuR phosphorylation by PKC may similarly change the abundance of these accessory factors and thus affect the bidirectional transport of HuR is a challenging topic that needs to be addressed by future studies.
Functionally, the PKC-dependent increase in cytosolic HuR abundance is linked to a substantial increase in COX-2 mRNA stability as we have ascertained in a human MC line stably overexpressing PKCα. Physiologically, the increase in COX-2 mRNA stability by ATP, which is paralleled by an amplification in PGE2 synthesis, may represent an important renovascular feedback mechanism activated by vasoactive agents (Stahl et al., 1984; Vonend et al., 2005). Such feedback mechanisms constitute a means by which the kidney increases the production of vasodilatory prostaglandins and thus counteracts a prolonged vasoconstriction and subsequent detrimental changes in the glomerular filtration rate. The discovery of the involvement of PKC in the posttranscriptional regulation of HuR shuttling warrants further investigation to elucidate its critical contribution to posttranscriptional gene expression controlled by HuR.
ACKNOWLEDGMENTS
We thank Henry Furneaux for kindly providing the plasmid pGEX-HuR. This work was supported by the Deutsche Forschungsgemeinschaft Grants EB 257/2-1, EB 257/2-2, PF 361/2-2, FOG 784, and EXC 147/1.
Abbreviations used:
- AMPK
AMP-activated kinase
- ARE
adenosine uridine-rich element
- COX
cyclooxygenase
- ELAV
embryonic lethal abnormal vision
- EMSA
electrophoretic mobility shift assay
- MC
human mesangial cell(s)
- IL
interleukin
- JNK
c-Jun NH2-terminal kinase
- MAPK
mitogen-activated protein kinase
- TNF
tumor necrosis factor
- UTR
untranslated region.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-09-0850) on March 28, 2007.
REFERENCES
- Akool E. S., Kleinert H., Hamada F. M., Abdelwahab M. H., Forstermann U., Pfeilschifter J., Eberhardt W. Nitric oxide increases the decay of matrix metalloproteinase 9 mRNA by inhibiting the expression of mRNA-stabilizing factor HuR. Mol. Cell. Biol. 2003;23:4901–4916. doi: 10.1128/MCB.23.14.4901-4916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A., Franzen R., Shabahang S., Fabbro D., Pfeilschifter J., Huwiler A. Ceramide induces translocation of protein kinase C-alpha to the Golgi compartment of human embryonic kidney cells by interacting with the C2 domain. Biochim. Biophys. Acta. 2003a;1634:30–39. doi: 10.1016/j.bbalip.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Aschrafi A., Shabahang S., Pfeilschifter J., Huwiler A. Regulatory functions of protein kinase C isoenzymes in the kidney. Curr. Top. Biochem. Res. 2003b;5:27–41. [Google Scholar]
- Chen C. Y., Shyu A. B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 1995;20:465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Dixon D. A., Tolley N. D., King P. H., Nabors L. B., McIntyre T. M., Zimmerman G. A., Prescott S. M. Altered expression of the mRNA stability factor HuR promotes cyclooxygenase-2 expression in colon cancer cells. J. Clin. Investig. 2001;108:1657–1665. doi: 10.1172/JCI12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G., Kim V. N., Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 2002;3:195–205. doi: 10.1038/nrm760. [DOI] [PubMed] [Google Scholar]
- Eberhardt W., Schulze M., Engels C., Klasmeier E., Pfeilschifter J. Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: involvement of nuclear factor-κB and Ets transcription factors. Mol. Endocrinol. 2002;16:1752–1766. doi: 10.1210/me.2001-0278. [DOI] [PubMed] [Google Scholar]
- Fan X. C., Steitz J. A. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sci. USA. 1998a;95:15293–15298. doi: 10.1073/pnas.95.26.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X. C., Steitz J. A. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998b;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Yang X., Wang W., Wood W. H., 3rd, Becker K. G., Gorospe M. Global analysis of stress-regulated mRNA turnover by using cDNA arrays. Proc. Natl. Acad. Sci. USA. 2002;99:10611–10616. doi: 10.1073/pnas.162212399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faour W. H., He Y., He Q. W., de Ladurantaye M., Quintero M., Mancini A., Di Battista J. A. Prostaglandin E(2) regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1β-treated human synovial fibroblasts. J. Biol. Chem. 2001;276:31720–31731. doi: 10.1074/jbc.M104036200. [DOI] [PubMed] [Google Scholar]
- Gallouzi I. E., Brennan C. M., Steitz J. A. Protein ligands mediate the CRM1-dependent export of HuR in response to heat shock. RNA. 2001;7:1348–1361. doi: 10.1017/s1355838201016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiges D., Meyer T., Marte B., Vanek M., Weissgerber G., Stabel S., Pfeilschifter J., Fabbro D., Huwiler A. Activation of protein kinase C subtypes α, γ, δ, ε, ζ, and η by tumor-promoting and nontumor-promoting agents. Biochem. Pharmacol. 1997;53:865–875. doi: 10.1016/s0006-2952(96)00885-4. [DOI] [PubMed] [Google Scholar]
- Good P. J. A conserved family of elav-like genes in vertebrates. Proc. Natl. Acad. Sci. USA. 1995;92:4557–4561. doi: 10.1073/pnas.92.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollams E. M., Giles K. M., Thomson A. M., Leedman P. J. mRNA stability and the control of gene expression: implications for human disease. Neurochem. Res. 2002;27:957–980. doi: 10.1023/a:1020992418511. [DOI] [PubMed] [Google Scholar]
- Huwiler A., Akool E. S., Aschrafi A., Hamada F. M., Pfeilschifter J., Eberhardt W. ATP potentiates interleukin-1β-induced MMP-9 expression in mesangial cells via recruitment of the ELAV protein HuR. J. Biol. Chem. 2003;278:51758–51769. doi: 10.1074/jbc.M305722200. [DOI] [PubMed] [Google Scholar]
- Huwiler A., Pfeilschifter J. Stimulation by extracellular ATP and UTP of the mitogen-activated protein kinase cascade and proliferation of rat renal mesangial cells. Br. J. Pharmacol. 1994;113:1455–1463. doi: 10.1111/j.1476-5381.1994.tb17160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A., Rolz W., Dorsch S., Ren S., Pfeilschifter J. Extracellular ATP and UTP activate the protein kinase B/Akt cascade via the P2Y(2) purinoceptor in renal mesangial cells. Br. J. Pharmacol. 2002;136:520–529. doi: 10.1038/sj.bjp.0704748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A., van Rossum G., Wartmann M., Pfeilschifter J. Stimulation by extracellular ATP and UTP of the stress-activated protein kinase cascade in rat renal mesangial cells. Br. J. Pharmacol. 1997;120:807–812. doi: 10.1038/sj.bjp.0700979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang B. C., Munoz-Najar U., Paik J. H., Claffey K., Yoshida M., Hla T. Leptomycin B, an inhibitor of the nuclear export receptor CRM1, inhibits COX-2 expression. J. Biol. Chem. 2003;278:2773–2776. doi: 10.1074/jbc.C200620200. [DOI] [PubMed] [Google Scholar]
- Li H., Park S., Kilburn B., Jelinek M. A., Henschen-Edman A., Aswad D. W., Stallcup M. R., Laird-Offringa I. A. Lipopolysaccharide-induced methylation of HuR, an mRNA-stabilizing protein, by CARM1. Coactivator-associated arginine methyltransferase. J. Biol. Chem. 2002;277:44623–44630. doi: 10.1074/jbc.M206187200. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma W. J., Cheng S., Campbell C., Wright A., Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- Ming X. F., Stoecklin G., Lu M., Looser R., Moroni C. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol. Cell Biol. 2001;21:5778–5789. doi: 10.1128/MCB.21.17.5778-5789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misquitta C. M., Iyer V. R., Werstiuk E. S., Grover A. K. The role of 3′-untranslated region (3′-UTR) mediated mRNA stability in cardiovascular pathophysiology. Mol. Cell Biochem. 2001;224:53–67. doi: 10.1023/a:1011982932645. [DOI] [PubMed] [Google Scholar]
- Moore M. J., Rosbash M. TAPping into mRNA export. Science. 2001;294:1841–1842. doi: 10.1126/science.1067676. [DOI] [PubMed] [Google Scholar]
- Nagy E., Rigby W. F. Glyceraldehyde-3-phosphate dehydrogenase selectively binds AU-rich RNA in the NAD(+)-binding region (Rossmann fold) J. Biol. Chem. 1995;270:2755–2763. doi: 10.1074/jbc.270.6.2755. [DOI] [PubMed] [Google Scholar]
- Pascale A., Amadio M., Scapagnini G., Lanni C., Racchi M., Provenzani A., Govoni S., Alkon D. L., Quattrone A. Neuronal ELAV proteins enhance mRNA stability by a PKCα-dependent pathway. Proc. Natl. Acad. Sci. USA. 2005;102:12065–12070. doi: 10.1073/pnas.0504702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavenstadt H., Gloy J., Leipziger J., Klar B., Pfeilschifter J., Schollmeyer P., Greger R. Effect of extracellular ATP on contraction, cytosolic calcium activity, membrane voltage and ion currents of rat mesangial cells in primary culture. Br. J. Pharmacol. 1993;109:953–959. doi: 10.1111/j.1476-5381.1993.tb13713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. S., Chen C. Y., Xu N., Shyu A. B. RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J. 1998;17:3461–3470. doi: 10.1093/emboj/17.12.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Huwiler A. Regulatory functions of protein kinase C isoenzymes in purinoceptor signaling in mesangial cells. J. Auton. Pharmacol. 1996;16:315–318. doi: 10.1111/j.1474-8673.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Radeke H. H., Meier B., Topley N., Floge J., Habermehl G. G., Resch K. Interleukin 1-β and tumor necrosis factor-α induce oxygen radical production in mesangial cells. Kidney Int. 1990;37:767–775. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- Rebane A., Aab A., Steitz J. A. Transportins 1 and 2 are redundant nuclear import factors for hnRNP A1 and HuR. RNA. 2004;10:590–599. doi: 10.1261/rna.5224304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. Control of messenger RNA stability in higher eukaryotes. Trends Genet. 1996;12:171–175. doi: 10.1016/0168-9525(96)10016-0. [DOI] [PubMed] [Google Scholar]
- Schulze-Lohoff E., Hugo C., Rost S., Arnold S., Gruber A., Brune B., Sterzel R. B. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am. J. Physiol. 1998;275:F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- Sengupta S., Jang B. C., Wu M. T., Paik J. H., Furneaux H., Hla T. The RNA-binding protein HuR regulates the expression of cyclooxygenase-2. J. Biol. Chem. 2003;278:25227–25233. doi: 10.1074/jbc.M301813200. [DOI] [PubMed] [Google Scholar]
- Stahl R.A., Helmchen U., Paravicini M., Ritter L.J., Schollmeyer P. Glomerular prostaglandin formation in two-kidney, one-clip hypertensive rats. Am. J. Physiol. 1984;247:F975–981. doi: 10.1152/ajprenal.1984.247.6.F975. [DOI] [PubMed] [Google Scholar]
- Vonend O., Okonek A., Stegbauer J., Habbel S., Quack I., Rump L. Renovascular effects of sympathetic cotransmitters ATP and NPY are age-dependent in spontaneously hypertensive rats. Cardiovasc. Res. 2005;66:345–352. doi: 10.1016/j.cardiores.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Wang W. J., et al. AMP-activated kinase regulates cytoplasmic HuR. Mol. Cell Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Yang X., Kawai T., Lopez de Silanes I., Mazan-Mamczarz K., Chen P., Chook Y. M., Quensel C., Kohler M., Gorospe M. AMP-activated protein kinase-regulated phosphorylation and acetylation of importin α 1, involvement in the nuclear import of RNA-binding protein HuR. J. Biol. Chem. 2004;279:48376–48388. doi: 10.1074/jbc.M409014200. [DOI] [PubMed] [Google Scholar]
- Winzen R., Kracht M., Ritter B., Wilhelm A., Chen C. Y., Shyu A. B., Muller M., Gaestel M., Resch K., Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. EMBO J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]