Abstract
The C haperonin Containing Tcp1 (CCT) maintains cellular protein folding homeostasis in the eukaryotic cytosol by assisting the biogenesis of many proteins, including actins, tubulins, and regulators of the cell cycle. Here, we demonstrate that the essential and conserved eukaryotic phosducin-like protein 2 (PhLP2/PLP2) physically interacts with CCT and modulates its folding activity. Consistent with this functional interaction, temperature-sensitive alleles of Saccharomyces cerevisiae PLP2 exhibit cytoskeletal and cell cycle defects. We uncovered several high-copy suppressors of the plp2 alleles, all of which are associated with G1/S cell cycle progression but which do not appreciably affect cytoskeletal protein function or fully rescue the growth defects. Our data support a model in which Plp2p modulates the biogenesis of several CCT substrates relating to cell cycle and cytoskeletal function, which together contribute to the essential function of PLP2.
INTRODUCTION
Phosducin-like proteins (PhLPs) are a conserved family of small thioredoxin-like proteins that were originally identified as modulators of heterotrimeric G protein signaling in the retina (Schroder and Lohse, 1996). Subsequently, they were shown to have roles in G protein signaling in other cell types as well as having G protein-independent functions (Flanary et al., 2000; Blaauw et al., 2003). One of the other functions of PhLPs is the regulation of the eukaryotic protein-folding machine known as Chaperonin Containing Tcp1 (CCT; also called TCP1-containing Ring Complex [TRiC]) (McLaughlin et al., 2002; Martín-Benito et al., 2004; Lukov et al., 2005, 2006; Stirling et al., 2006).
Chaperonins are oligomeric molecular chaperones that bind nonnative proteins and facilitate their transition to the native state (Hartl and Hayer-Hartl, 2002). These barrel-shaped molecular machines undergo large conformational changes to encapsulate and release bound substrate proteins during their ATP-dependent folding cycle (Spiess et al., 2004). In the eukaryotic cytosol, the chaperonin CCT ensures the correct folding and assembly of a variety of proteins. The best-characterized substrates of CCT are actins and tubulins, although several recent studies have extended the number of known CCT substrates, including several cell cycle proteins (Thulasiraman et al., 1999; Camasses et al., 2003; Siegers et al., 2003; for review, see Spiess et al., 2004). CCT cooperates with another chaperone called Prefoldin (PFD) in the folding of actins and tubulins (Geissler et al., 1998; Vainberg et al., 1998). PFD uses six long coiled-coil “tentacles” to stabilize substrate proteins at the opening of its jellyfish-shaped cavity before their delivery to the open CCT cavity (Vainberg et al., 1998; Siegers et al., 1999; Siegert et al., 2000; Lundin et al., 2004). Together, PFD and CCT compose a folding pathway for cytoskeletal proteins, which, along with PhLPs, control the folding of actin and tubulin (Siegers et al., 1999; Lacefield and Solomon, 2003; Stirling et al., 2006).
PhLPs can be subdivided into three homologous families, called PhLP1, PhLP2, and PhLP3, that share an N-terminal helical domain, a central thioredoxin-like fold, and a charged C-terminal extension (Blaauw et al., 2003). PhLP1 proteins are the best characterized and function both in G protein signaling and the regulation of CCT (Schroder and Lohse, 1996; McLaughlin et al., 2002; Martín-Benito et al., 2004; Lukov et al., 2005, 2006). PhLP3 proteins act as modulators of CCT and play roles in actin and tubulin biogenesis in yeast and microtubule function in Caenorhabditis elegans (Lacefield and Solomon, 2003; Ogawa et al., 2004; Stirling et al., 2006). PhLP2 proteins are the least characterized PhLP isoforms, but they are essential in Dictyostelium discoideum and possibly also Saccharomyces cerevisiae (Flanary et al., 2000; Blaauw et al., 2003). PhLP2 may act as a regulator of apoptosis in mammalian cells, but the significance and mechanism of this function is unclear (Wilkinson et al., 2004).
Yeast possess homologues of PhLP3 and PhLP2, which are encoded by PLP1 and PLP2, respectively (Blaauw et al., 2003). PLP1/PhLP3 has been implicated in CCT-mediated folding of actin and tubulin, and it is thought to work at the level of CCT to regulate the ATP-hydrolysis–dependent turnover of CCT–substrate complexes (Lacefield and Solomon, 2003; Stirling et al., 2006). Although PLP1 has an important function in regulating actin and tubulin folding, plp1Δ cells have no apparent growth defects. Indeed, most plp1Δ phenotypes are only detected in strains that also lack a functional PFD complex (Lacefield and Solomon, 2003; Stirling et al., 2006). Conversely, PLP2 is seemingly essential for growth, but its cellular function is thus far unknown (Flanary et al., 2000; Lopez et al., 2003).
To elucidate the essential function of PLP2, we undertook studies of yeast and human PhLP2 homologues. Yeast PLP2 binds to CCT as suggested by proteome-wide studies (Gavin et al., 2006). In this regard, we show that in vitro, human PhLP2A inhibits actin folding and forms ternary complexes with CCT and actin. We also show in yeast that temperature-sensitive (ts) alleles of PLP2 are defective in CCT-regulated processes such as actin and tubulin function, and cell cycle progression. plp2-ts alleles do not, however, exhibit altered sensitivity or resistance to α-factor, supporting the notion that regulating G protein signaling is not part of the essential function of Plp2p, as might be the case for certain mammalian phosducins (Schroder and Lohse, 1996). Finally, we identify high-copy suppressors of plp2-ts alleles that indicate an essential function for PLP2 during G1/S phase cell cycle progression. Our data support a model in which Plp2p modulates the biogenesis of several CCT substrates, which together contribute to the essential function of PLP2.
MATERIALS AND METHODS
Purification of GST-PhLP2A, and CCT
Human PhLP2A (accession no. AF110511) and PhLP3 (accession no. NM_005783) were cloned into the glutathione S-transferase (GST) fusion vector pGEX-6p, and expressed in BL21[DE3]pLysS as described previously (Stirling et al., 2006). GST-fusion proteins were purified with glutathione-Sepharose 4B as per manufacturer's instructions (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). GST alone, used as a control, was expressed and purified exactly as for GST-PhLP2A. CCT was purified from rabbit reticulocyte lysate as described previously (Gao et al., 1992; Melki et al., 1997).
In Vitro Translation and Folding Assays
Actin and tubulin were translated in the T7 quick coupled translation reaction (Promega, Madison, WI) according to manufacturer's instructions. Actin was also translated in Escherichia coli lysate by using the EcoPro system (Novagen, Madison, WI). Recombinant GST or GST-PhLP2A was added at ∼100 times excess to endogenous levels of CCT before translation (Cowan, 1998). At various time points, aliquots of the reaction were frozen in native gel running buffer and then thawed on ice and analyzed by native gel electrophoresis (Leroux, 2000). GST-pull-downs were performed with glutathione-Sepharose 4B beads according to manufacturer's instructions (GE Healthcare). For GST pull-downs from reticulocyte lysate and E. coli lysate translation reactions (Figure 6, B and C), the translation reaction (with or without exogenous CCT) was diluted 100 times into phosphate-buffered saline containing GST or GST-PhLP2A before precipitating the GST-fusions as described above. Mammalian Tcp1 was detected using a rat monoclonal antibody directed against a C-terminal fragment of mouse Tcp1α (Assay Designs, Ann Arbor, MI).
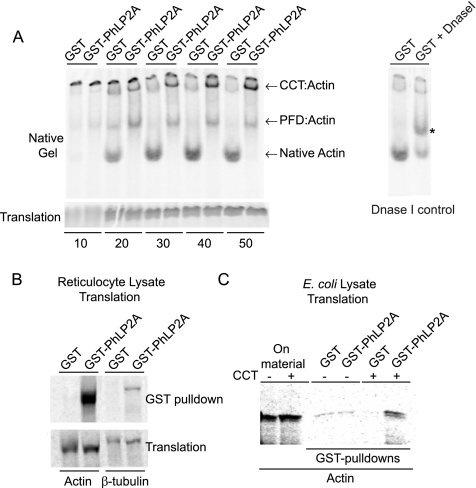
Figure 6.
Mammalian PhLP2A modulates CCT-mediated actin folding in vitro. (A) In vitro folding reactions of nascent 35S-labeled actin in the presence of GST or GST-PhLP2A and separated on a nondenaturing gel at the time points (minutes) indicated. CCT:actin and PFD:actin binary complexes, as well as native actin, are indicated. The identity of the fast-migrating band as native monomeric actin was verified by Dnase I-shifting on the native gel (right). Asterisk (*) indicates Dnase I bound native actin. Note the reduction in the intensity of the bottom band. Note the decrease in native actin production over time in the presence of GST-PhLP2A but not GST alone (see Supplemental Figure S3 for quantitation of band intensities). An SDS-gel illustrating the relative amounts of translation products is shown (bottom). (B) Coprecipitation of 35S-labeled actin or β-tubulin with GST-PhLP2A in a reticulocyte translation reaction. The lower panel shows that the levels of translation were comparable. (C) Coprecipitation of 35S-labeled actin produced in E. coli lysate takes place only in the presence of GST-PhLP2A and exogenously added CCT, indicating the formation of a ternary complex. Actin cDNA was translated in E. coli lysate with or without the addition of purified rabbit CCT, and the relative levels of the translation products are shown on the left. GST-PhLP2A coprecipitated the newly-made actin only in reactions to which CCT had been added, whereas GST alone had no such affect as shown on the right.
Yeast Strains, Media, and Growth Assays
For yeast strains and plasmids, see Supplemental Tables S1 and S2. Yeast were grown on YEPD, or synthetic complete media as required (Adams et al., 1997). 5′-Fluoroorotic acid plates were also made as described previously (Adams et al., 1997). For temperature-sensitive growth assays, log-phase cells were serially diluted 10-fold, spotted on the appropriate media, and cultured at the temperatures indicated. To assess the reversibility of the ts-alleles, the indicated strains were cultured at the restrictive temperature for the times shown, and plating efficiency was observed directly under the microdissection microscope as described previously (Amberg et al., 2005).
Plp2p, CCT Coimmunoprecipitation
For immunoprecipitation–immunoblotting experiments, a strain bearing integrated myc-tagged PLP2 and hemagglutinin (HA)-tagged CCT2 (AHY994), or control strains lacking the tagged PLP2, or both tagged proteins, were grown to mid-log phase. Extracts were prepared as described previously (Zachariae et al., 1998) from 2 × 106 cells in 0.4 ml of buffer B70 (number indicates millimolar potassium acetate). Cleared extracts (0.35 ml or 4 mg) were incubated for 60 min with antibodies, which were captured with 40 μl of protein A-Sepharose for 60 min as described previously (Camasses et al., 2003). Beads were washed with the buffers B70 plus bovine serum albumin (1 mg/ml), B70, B150, B200, and B70 followed by immunoblot analysis.
Drug and Mating Factor Sensitivity Assays
Latrunculin B (LatB; Sigma-Aldrich, St. Louis, MO) sensitivity assays were performed as described previously (Ayscough et al., 1997). Benomyl or mating factor sensitivity assays were performed essentially as described for LatB. Briefly, sterile filter paper disks were soaked with the drug or pheromone at the concentration indicated and placed on a soft agar overlay containing a particular yeast strain. The radius of clearance around the disk was measured after 2 d of growth at 30°C. Congenic control strains carrying wild-type copies of the genes under examination were included for each experiment.
Generation of Temperature-sensitive Alleles of PLP2
A plp2 null strain was made by HIS3 gene insertion into the PLP2 coding region. To generate the plp2-ts mutants, the PLP2 gene (including 1 kb of the upstream promoter and 120 base pairs after the stop codon) was cloned into the pRS405 Leu vector. plp2 mutations were generated within the coding region by error-prone polymerase chain reaction (PCR) (0.3 mM MnCl2, 0.5 mM dCTP, 0.5 mM dTTP, 0.1 mM dGTP, 0.1 mM dATP, and wild-type Taq polymerase for 28 cycles) and the wild-type PLP2 opening reading frame was replaced via gap-repair. Mutagenized plasmids able to rescue the plp2 null strain were then screened for temperature sensitivity. Plasmids from temperature-sensitive transformants were sequenced, retransformed, and integrated into AHY955 with a LEU2 marker before counterselection of the URA3-marked PLP2 plasmid with 5′-fluoroorotic acid (Supplemental Table S1). The plp2-1 strain contained the mutations: R24/S24, Q91/L91, Q149/R149, V191/A191, L207/Q207, and S259/T259. The plp2-2 strain contained the mutations: Q91/R91, D132/V132, F164/S164, N175/D175, and E183/G183.
Microscopy
Yeast strains were grown to mid-log phase in YPD or SC media before direct imaging or fixing and staining. Tetramethylrhodamine isothiocyanate-conjugated phalloidin (Sigma-Aldrich) and 4′,6-diamidino-2-phenylindole (DAPI) were used, according to manufacturer's instructions, to stain formaldehyde fixed yeast cells as described previously (Adams et al., 1997). Calcofluor white staining was carried out as described in Amberg et al. (2005). Actin immunofluorescence was performed as described by Adams et al. (1997). For cell sizing, at least 100 cell diameters were measured in Openlab 5.0.2 (Improvision, Coventry, United Kingdom) for each strain perpendicular to the mother-bud axis at the largest point. Cellular defects were statistically validated using an independent variable t test or chi-square analysis, as appropriate.
High-Copy Suppression Screen
A YEp24-based library of URA3-marked plasmids containing ∼10-kb fragments of S. cerevisiae genomic DNA (Carlson and Botstein, 1982) was transformed into plp2-1 and plp2-2 cells. Half of the cells were plated at a permissive temperature (29°C) to calculate transformation efficiency and half were plated at the nonpermissive temperature (37°C) to identify suppressing clones. Colonies which grew at 37°C after 48 h were cultivated and the plasmid DNA was isolated and screened by PCR to determine whether it contained a copy of PLP2. Plasmid inserts lacking PLP2 were sequenced at both ends and genes therein were identified using the yeast genome database (www.yeastgenome.org). Plasmids bearing some of the individual genes identified were a kind gift from Dr. Joaquín Ariño (Universidad Autonoma de Barcelona, Barcelona, Spain) (Muñoz et al., 2003).
Cell Synchronization
For fluorescence activated cell sorting (FACS) analysis, cells were synchronized as small G1 cells by centrifugal elutriation as described by Schwob and Nasmyth (1993). After elutriation, samples were incubated at 37°C, and, at 15-min intervals, stained for DNA content with propidium iodide. All samples were analyzed on a FACScan (BD Biosciences, San Jose, CA) as described previously (Epstein and Cross, 1992). For rebudding analyses, cells were synchronized with α-factor according to the low pH method (Amberg et al., 2005). Synchronized cells were shifted to 37°C for 1 h before release into 37°C media. Time points were taken after release and the budding index of the culture scored.
RESULTS
PLP2 Is an Essential CCT-binding Protein
Previously, we found that Plp1p, the yeast PhLP3 homologue, cooperates with CCT and PFD to modulate actin and tubulin function in vivo (Stirling et al., 2006). We initially hypothesized that the only other yeast phosducin-like protein, Plp2p, might function similarly to modulate CCT activity. However, several lines of evidence suggest that the Plp1 and Plp2 proteins are unlikely to have identical functions in vivo. Yeast lacking PLP1 are viable, whereas yeast lacking PhLP2 (PLP2) do not survive. In addition, overexpression of PLP1 cannot complement the deletion of PLP2 (Flanary et al., 2000).
To determine whether S. cerevisiae Plp2p influences CCT-mediated protein folding, we first tested whether Plp2p interacts with CCT in vivo. When myc-tagged Plp2p was coexpressed with HA-tagged Cct2p, Cct2p coprecipitated with myc-Plp2p by using an anti-myc antibody, indicating a potentially direct physical interaction between the two proteins (Figure 1A). This result is consistent with previous proteome-scale TAP-tagging studies that identified an interaction between six of eight CCT subunits and Plp2p, suggesting that the entire CCT holocomplex interacts with Plp2p (Gavin et al., 2006). To assess whether this interaction is conserved in mammalian cells, we purified a GST-tagged human PhLP2A fusion protein for use in in vitro experiments. Similar to PhLP3, purified CCT coprecipitated with the human GST-fused PhLP2A, but not GST alone, indicating that PhLP2A and CCT form a complex (Figure 1B; Stirling et al., 2006). Given that both native PhLP3 and PhLP1 form complexes with CCT (McLaughlin et al., 2002; Stirling et al., 2006), our results with Plp2p/PhLP2A now confirm that all known phosducin-like proteins (except phosducin itself) interact with CCT and do so not as substrates, but rather as native binding partners (McLaughlin et al., 2002).
Figure 1.
PhLP2/PLP2 is an essential CCT-binding protein. (A) CCT2-HA is immunoprecipitated using an anti-myc antibody from S. cerevisiae cells coexpressing CCT-HA and plp2-myc but not from control wild-type (WT) or CCT-HA–expressing cells. HA-tagged CCT is detected by Western blot analysis by using an anti-HA antibody. (B) Western blot showing CCT coprecipitating in vitro with GST-PhLP2A or GST-PhLP3 in a GST pull-down, but not the GST-alone control. Purified CCT was run as a control to show that the antibody recognizes multiple CCT subunits (left). (C) Counterselection of a URA3-marked plasmid carrying PLP2 ([pURA3 PLP2]) in plp2Δ cells with 5′-fluoroorotic acid (5′FOA) shows that PLP2 is an essential gene. Cells prototrophic (PLP2 URA3) and auxotrophic (PLP2 ura3-52) for uracil (ura) were included as controls. SC, synthetic complete media.
There has been some dispute as to whether PLP2 is truly essential for growth or is instead required for spore germination (Flanary et al., 2000; Lopez et al., 2003). To clarify this discrepancy, we generated a haploid yeast strain lacking the chromosomal copy of PLP2, and we transformed it with a URA3-marked plasmid containing PLP2. When cultured on medium containing the drug 5′-fluoroorotic acid, which selects against cells carrying a functional copy of the URA3 gene, no growth was observed (Figure 1C). Although it is still possible that PLP2 is essential in only some strain backgrounds, this experiment demonstrates that some yeast cannot survive without PLP2.
Generation of PLP2 Temperature-sensitive Alleles
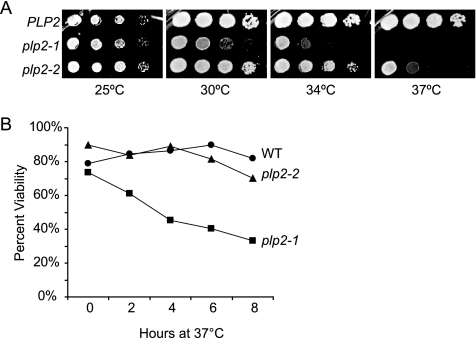
To study the effects of PLP2 loss-of-function, we generated temperature-sensitive alleles of PLP2 by error-prone PCR mutagenesis of the wild-type gene. Two of the alleles identified were chosen for further characterization, namely, plp2-1, the most severe allele, and plp2-2, a less severe allele (Figure 2A). The plp2-1 mutant exhibits growth defects at 30°C, whereas plp2-2 cells are only slightly impaired for growth at 34°C. Both mutant alleles cause lethality at 37°C. The two alleles contain multiple sequence changes (see Materials and Methods), but interestingly, both are mutated at Q91, which aligns to the region at the C terminus of helix 3 between the N-terminal and the thioredoxin domains (Gaudet et al., 1996; Blaauw et al., 2003). To determine whether the ts-phenotype is reversible, we cultured wild-type, plp2-1, and plp2-2 cells at 37°C, and then we microdissected arrested cells onto solid medium and incubated them at 25°C, a permissive temperature of growth for all strains. Figure 2B shows that the temperature sensitivity is not reversible for plp2-1 cells after 4 h at 37°C, because >50% of the cells are inviable. In contrast, the temperature sensitivity of plp2-2 cells is largely reversible, because even after 8 h at 37°C, 70% of the cells form colonies after shifting to the permissive temperature (Figure 2B). Thus, phenotypes observed in plp2-2 cells at 37°C do not reflect general necrotic defects, but rather they indicate specific cellular defects caused by PLP2 loss-of-function.
Figure 2.
Generation and analysis of temperature-sensitive plp2 alleles. (A) Wild-type yeast (PLP2), or two temperature-sensitive alleles of PLP2, namely, plp2-1 and plp2-2, were spotted on YPD media and grown at the temperatures shown for 48 h. plp2-1 displays a more severe ts phenotype than plp2-2. (B) Reversibility of the plp2-ts alleles was assessed after growth at 37°C for the times shown. Shifted cells were plated at a permissive temperature and the number of microcolony forming cells was assessed under a microdissection microscope. plp2-2 temperature sensitivity is largely reversible compared with the more severe plp2-1 allele.
plp2-ts Alleles Exhibit Cytoskeletal but Not G Protein-related Defects
Given the putative role of yeast phosducin-like proteins as negative regulators of heterotrimeric G protein signaling, we tested the sensitivity of plp2–ts strains to mating pheromone, which is a readout of yeast G protein activation. Consistent with previous data (Flanary et al., 2000), we saw no additional sensitivity to pheromone compared with yeast carrying wild-type PLP2 (Supplemental Figure S1). Although it is possible that more subtle effects on pheromone signaling were overlooked, these data support an essential role for Plp2p outside of mating pheromone signaling and possibly relating to CCT function (Flanary et al., 2000; Gavin et al., 2006).
Although CCT impacts a variety of substrate proteins, its most abundant and best characterized substrates are the cytoskeletal proteins actin and tubulin, and, accordingly, yeast ts alleles of CCT display various actin- and tubulin-related cytoskeletal defects (Gao et al., 1992; Ursic et al., 1994; Vinh and Drubin, 1994; Siegers et al., 1999; Spiess et al., 2004). We therefore predicted that if Plp2p modulates the function of CCT, by way of direct physical interaction (Figure 1, A and B), the plp2–ts alleles might also exhibit cytoskeletal anomalies. To investigate this possibility, we first tested the sensitivity of plp2–ts alleles to actin and microtubule-disrupting drugs. Figure 3A shows that plp2–ts alleles exhibit sensitivity to both latrunculin and benomyl, indicating microfilament and microtubule defects, respectively. Similar phenotypes are also observable in strains carrying ts or cold-sensitive (cs) alleles of several CCT genes (our unpublished observations; Ursic et al., 1994; Vinh and Drubin, 1994).
Figure 3.
plp2-ts cells have increased sensitivity to cytoskeletal-destabilizing drugs and are larger than wild-type cells. (A) Benomyl and latrunculin sensitivity of plp2-1 and plp2-2 mutants relative to wild-type (PLP2) cells, as determined by relative clearance caused by drug-inoculated paper discs when grown at the semipermissive temperature of 30°C. (B) Cell sizes of the indicated strains were measured perpendicular to the mother-daughter axis for at least 100 cells. An asterisk (*) indicates that, relative to wild type, the cells were significantly larger (p < 0.01) as determined by an independent variable t test.
Under the microscope, plp2–ts cells also exhibited morphological phenotypes consistent with actin and tubulin cytoskeleton defects. When cultured at the nonpermissive temperature of 37°C, plp2–ts cells became larger (Figure 3B), a possible indication of defects in actin filament organization (Drubin et al., 1993). Indeed, plp2-1 cells were significantly larger than their wild-type counterparts even at permissive temperatures (Figure 3B). Moreover, after 8 h of growth at 37°C, the plp2–ts cells exhibited budding defects such as multiple buds (0% wild type, 7% plp2-1, 30% plp2-2; p < 0.01; Supplemental Table S3) and a thickening of the bud-neck junction between mother and daughter cells (0% wild-type, 11% plp2-1, 26% plp2-2; p < 0.001; Supplemental Table S4). These types of budding defects are also consistent with disrupted actin function or organization (Drubin et al., 1993).
Finally, we observed chitin organization defects in plp2-ts cells as well as in several CCT mutant strains as assessed by calcofluor white staining (Supplemental Figure S2). Chitin defects have been observed before for specific actin mutants, consistent with cytoskeletal defects in our mutant strains (Drubin et al., 1993). Interestingly, no such defects were observed in deletions of either the prefoldin subunit gene PAC10 or the phosducin-like gene PLP1, suggesting a CCT/PLP2-specific defect (Supplemental Figure S2).
Microtubule and Nuclear Defects in plp2-ts Alleles
Microtubules are essential for orienting the mitotic spindle and for proper segregation of chromosomes during anaphase. The improper segregation of a nucleus to the daughter bud, therefore, can be an indication of microtubule defects. Such a phenotype can be observed, for example, by abrogating prefoldin or CCT function (Ursic et al., 1994; Lacefield and Solomon, 2003; Stirling et al., 2006). We stained nuclei in plp2-ts cells with DAPI to examine possible defects in nuclear segregation. Compared with wild-type cells, a significant (p < 0.01) number of unbudded (11%), small-budded (11%), and large-budded (22%) plp2-1 cells contained multiple nuclei when incubated at the restrictive temperature for 4 h (Figure 4A and Supplemental Table S5). Similarly, the multinucleate phenotype was found to be significant (p < 0.001) in small- and large-budded plp2-2 cells (16 and 23% respectively) at 37°C for 4 h. A statistically significant number of unbudded plp2-1 cells were also anucleate at both permissive and nonpermissive temperatures, although the defect was more penetrant at high temperature (6 and 28%, respectively) (Figure 4A and Supplemental Table S5). Quantification of the number of cells going through anaphase revealed that both plp2-1 and plp2-2 mutant cells had significantly fewer cells in anaphase than wild-type cells at the nonpermissive temperature (37% wild-type, 22% plp2-1, and 17% plp2-2 large-budded cells in anaphase; Supplemental Table S6). This type of defect is consistent with the activation of a cell cycle checkpoint, possibly in response to incorrect anaphase spindle positioning.
Figure 4.
plp2-ts cells exhibit aberrant nuclear segregation and spindle orientation. (A) Images of DAPI-stained PLP2, plp2-1 and plp2-2 cells grown at permissive (23°C) and nonpermissive (37°C) temperatures. Arrows indicate multinucleate cells. (B) GFP-α-tubulin–expressing PLP2, plp2-1, and plp2-2 cells were visualized and scored for spindle orientation with respect to the mother-daughter axis. Cells are outlined in white and the percentages of misoriented spindles are indicated below each panel. Representative images are shown of normal spindles for wild-type and plp2-1 and plp2-2 cells at 23°C and of misoriented spindles in plp2-1 and plp2-2 at 37°C.
One possible explanation for the observed benomyl sensitivity and DNA segregation defects of the plp2–ts alleles is a deficiency in tubulin cytoskeleton function. We therefore expressed a green fluorescent protein (GFP)-α-tubulin (Tub1p) fusion protein in wild-type, plp2-1, and plp2-2 cells to assess the integrity of microtubules. Interestingly, we observed superficially normal microtubules in the plp2 mutant cells incubated at the nonpermissive temperature of 37°C for 4 h (data not shown). Considering that aberrant spindle positioning could also lead to segregation defects, we speculated that the benomyl sensitivity and defects in nuclear segregation may result from errors in spindle positioning, and not spindle assembly or tubulin defects per se. When we examined the orientation of spindles with respect to the mother-bud axis, we found that 45 and 42% (p < 0.001) of plp2-1 and plp2-2 cells, respectively, had clearly misoriented mitotic spindles (Figure 4B and Supplemental Table S7). Because actin function is required for establishing the correct orientation of the mitotic spindle in the G1 phase of the cell cycle (Theesfeld et al., 1999), these data could in principle explain both the latrunculin and benomyl sensitivity observed (Figure 3A). Whether these defects relate solely to actin dysfunction or whether tubulin also plays a role remains unclear.
Actin Polarization Defects in plp2-ts Alleles
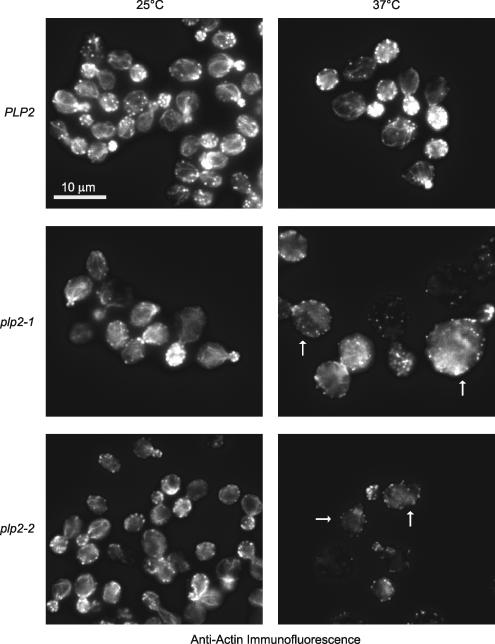
To better understand any actin cytoskeletal defects in cells with defects in PLP2 function, we stained the actin cytoskeleton with anti-actin antibodies (see Materials and Methods; Figure 5). When examined by immunofluorescence, actin localization in the plp2–ts mutants was essentially normal at 25°C, but after shifting to the restrictive temperature of 37°C for 4 h, severe defects were observed in both plp2-1 and plp2-2 cells (Figure 5 and Supplemental Table S8). After incubation at 37°C, actin cables were absent in a very large proportion of unbudded plp2-1 (93%) and plp2-2 cells (86%), small-budded plp2-1 (72%) and plp2-2 cells (94%) and large-budded plp2-1 (90%) and plp2-2 cells (91%) (Figure 5 and Supplemental Table S8). In plp2-1 and plp2-2 cells, the polarization of the actin cytoskeleton was also defective (Figure 5 and Supplemental Table S8). In wild-type cells, cortical actin patches are polarized to the bud site in unbudded cells and toward the bud tip in small-budded cells. However, at 37°C, normal actin patch polarization in plp2-1 cells was observed in only 12% unbudded, 36% small-budded, and 10% large budded cells. In plp2-2 cells at 37°C, normal actin patch polarization was observed in only 6% unbudded, 30% small budded, and 7% large-budded cells. A significant proportion of unbudded plp2-1 cells also exhibited abnormal actin patch polarization even at 25°C (Supplemental Table S8). Importantly, these data also support our observed functional cooperation between Plp2p and CCT in actin folding, because mutations in CCT or PFD subunits show similar actin organization defects (Ursic et al., 1994; Vinh and Drubin, 1994; Geissler et al., 1998; Vainberg et al., 1998).
Figure 5.
Actin filament organization defects in plp2-ts cells. (A) PLP2, plp2-1, and plp2-2 cells grown at permissive temperature (25°C) or nonpermissive temperature (37°C) were stained with anti-actin antibodies. Arrows indicate examples of cells lacking clear actin cables and having poorly polarized actin patches.
Mammalian PhLP2A Inhibits Actin Folding In Vitro and Binds CCT–Actin Complexes
Because disruption of PLP2 function seems to strongly affect the actin cytoskeleton in vivo, we exploited the robust in vitro actin folding system developed using reticulocyte lysate (Cowan, 1998; Vainberg et al., 1998; Leroux, 2000) to examine whether PhLP2 modulates the folding function of CCT. In this system, nascent [35S]methionine-radiolabeled actin is produced, and actin folding is examined on a native polyacrylamide gel (Leroux, 2000). Because mammalian actin, CCT, and other mammalian components have been well tested in this system, we used purified GST-fused human PhLP2A protein to test for its effect on actin folding. Figure 6A shows that in the presence of excess GST-PhLP2A, the production of native actin was greatly inhibited at all time points assayed, compared to GST alone. The presence of GST- PhLP2A also led to an increase in the amount of radiolabeled actin associated with both the CCT and PFD complexes (Figure 6A), whose positions on native gels and actin-binding activities are well established (Cowan, 1998; Vainberg et al., 1998; Leroux, 2000). Quantification of the native actin and chaperone:actin bands confirmed our qualitative conclusions that inhibition of folding was occurring and that nonnative actin was accumulating on CCT and PFD (Supplemental Figure S3). This result is consistent with PhLP2A slowing the reaction cycle or inhibiting the activity of CCT; this inhibition could potentially be taking place in a ternary complex with CCT and substrate, as reported for PhLP3, leading to a backlog of unfolded substrate that ends up bound to PFD, and, in this case, also CCT (Figure 6A; Lukov et al., 2006; Stirling et al., 2006).
We therefore examined whether, similar to that observed for PhLP3, PhLP2A could form ternary complexes with CCT and substrate (e.g., CCT-PhLP3-actin or CCT-PhLP3-tubulin; Stirling et al., 2006). To this end, we performed GST pull-downs of in vitro translation reactions of actin or tubulin containing exogenous GST-PhLP2A or GST alone as a negative control. Figure 6B shows in vitro translations of actin and β-tubulin in reticulocyte lysate, which contains endogenous CCT, that have been precipitated with glutathione-conjugated beads. GST-PhLP2A effectively precipitates both actin and β-tubulin, whereas GST alone has no such effect. To determine whether the interaction between PhLP2A and a cytoskeletal protein (actin) depends on CCT (as opposed to PhLP2A binding the substrate directly), we performed a similar experiment with an E. coli lysate translation system, which lacks CCT. We found that GST-PhLP2A only precipitates actin when exogenous, purified CCT was added (Figure 6C). Under these conditions, GST-PhLP2A is able to specifically recover CCT in the pull-down (data not shown). Together, these data suggest that PhLP2A negatively modulates CCT function in a manner that may be similar to that of the mammalian PhLP3 protein (Stirling et al., 2006).
High-Copy Suppression of plp2-ts Alleles Reveals Links to the G1/S Phase Transition
Our finding that Plp2p influences actin microfilament and tubulin microtubule function in S. cerevisiae is consistent with the fact that Plp2p associates with and affects the function of CCT, a chaperone essential for actin and tubulin folding. However, CCT has been implicated in the biogenesis of several other proteins, some of which are linked to the cell cycle. To take an unbiased approach to understanding the essential function(s) of PLP2, which may be CCT-dependent or (potentially) independent, we executed a high-copy suppression screen of the plp2–ts-alleles.
A high-copy plasmid library carrying ∼10-kb fragments of yeast genomic DNA (Carlson and Botstein, 1982) was transformed into the plp2-1 strain, and colonies were plated at 37°C. Plasmids isolated from colonies that grew well at 37°C were screened by PCR to exclude those carrying the PLP2 gene, a predictable suppressor. Plasmids that did not contain PLP2 were sequenced to identify the gene(s) present in the suppressing plasmid. One high-copy fragment we isolated contained PLC1, a yeast phospholipase C homologue. We confirmed that PLC1 was a suppressor because a galactose-inducible PLC1 construct could partially suppress both plp2-1 and plp2-2 alleles (Supplemental Figure S4). Another suppressing plasmid encoded two genes, VHS1 and PAM1. These were expressed individually in plp2-1 cells, and, surprisingly, both were found to partially suppress the temperature-sensitive defect (Figure 7A). In the same screen from which VHS1 was previously identified, several other genes, including protein phosphatase subunits, PAM1 and other genes active in the G1/S phase transition, were found to suppress loss of SIT4 and HAL3 (a phosphatase subunit and regulator, respectively). To try and identify additional plp2-1 suppressors, we tested some of the sit4-hal3 suppressors found in the screen by Muñoz et al. (2003) (Figure 7B). Remarkably, the genes uncovered, VHS2, VHS3, PTC2, PTC3, YAP7, HAL5, HAL3, and CLN3, were also able to partially suppress temperature-sensitive growth defects of plp2-1 cells. This apparent lack of specificity suggests that driving cells to continue through the cell cycle helps to somewhat alleviate the plp2-based temperature sensitivity. The extensive overlap between suppressors of plp2-ts alleles and suppressors of sit4-hal3 mutants (Muñoz et al., 2003) suggests either that Plp2p directly affects Sit4p and/or Hal3p or that Plp2p works in a parallel pathway to that of Sit4p and Hal3p. The absence of suppressors directly related to cytoskeleton functions may imply that these defects cannot be suppressed by overexpression of any one particular gene or that any suppression was too weak and thus overlooked.
Figure 7.
High-copy suppressors of plp2-1 indicate a role for PLP2 in cell cycle progression. Partial suppressors of plp2-1 temperature sensitivity identified in a high-copy screen (PLC1 suppression is shown in Supplemental Figure S4) (A) and suppressors identified in the literature (B). plp2-1 cells carrying the indicated plasmids were grown to log phase and serially diluted on SC-Ura media and grown for 2–3 d at permissive (25°C) or restrictive (37°C) temperatures before imaging. (C) A delay in DNA replication is observed in synchronized plp2-1 cells. Graphs indicate FACS analysis of cells stained for DNA content over time. (D) Allele specific suppression of plp2-ts alleles. Serially diluted plp2-2 cultures carrying the indicated plasmids were grown for 2–3 d on SC-Ura as in A and B before imaging.
Although the partial suppressors of plp2-1 have some diverse functions, many are involved in protein phosphorylation/dephosphorylation and they all play roles in promoting the G1/S phase transition of the cell cycle (Muñoz et al., 2003). These data therefore suggest that PLP2 also has a role in G1 progression. Indeed, we observed an accumulation of unbudded plp2-1 cells after 2 h at 37°C (50% unbudded, p < 0.001; Supplemental Table S9), although this could also be related to the actin defects reported above. Also, the observation that plp2–ts cells become larger than wild-type cells at 37°C is consistent with a pause in progression from G1 to S phase. To test these suppositions, we examined the entry of synchronized plp2-1 cells into S phase. Synchronized 1N cell populations were prepared by elutriation, grown at 37°C, stained at various time points for DNA content, and subjected to FACS. Figure 7C shows that plp2-1 cells are delayed in their entry into S phase compared with their wild-type counterparts. Even after 4 h, there is still a greater proportion of plp2-1 cells in G1 than G2, whereas approximately half of the wild-type cells are found in G2 (Figure 7C). This delay in cell cycle progression is consistent with the G1/S function of the plp2-1 suppressors we identified, and the accumulation of unbudded cells in asynchronous plp2-1 cell populations at restrictive temperatures (Supplemental Table S9). Consistent with these observations, we also observed a delay in budding when α-factor-synchronized plp2–ts cells were released in 37°C media (Supplemental Figure S5). Importantly, entry into S-phase seems to be independent of actin function (McMillan et al., 1998), and we found that the actin cytoskeleton appears similarly defective in plp2-1 and plp2-2 cells carrying high-copy empty vector, or the suppressing PTC2- or YAP7-plasmids (our unpublished observations). Our data therefore suggest an additional, actin-independent role for PLP2 in the cell cycle.
To explore the possibility of allele-specific suppression, we tested two of the stronger suppressors of plp2-1 (YAP7 and PTC2) in plp2-2 cells (Figure 7A). Although high-copy production of YAP7 suppresses plp2-2 temperature sensitivity, PTC2 overexpression actually inhibits the growth of plp2-2 cells (Figure 7D). Also consistent with functional differences between the plp2 alleles, unbudded cells did not accumulate at the restrictive temperature for plp2-2 as they did for plp2-1 (Supplemental Table S9). Finally, we have observed some semidominant growth defects for plp2-1 alleles not found in the plp2-2 strain (data not shown). Together, the observed allele specificity supports a role for PLP2 in the cell cycle because the cytoskeletal phenotypes of plp2-1 and plp2-2 cells are nearly identical (Figures 3–5). Indeed, if the observed cell cycle defects were somehow an indirect effect of cytoskeletal defects, the two plp2 alleles would be expected to behave similarly.
DISCUSSION
In recent years, the phosducin-like proteins PhLP1 and PhLP3 have emerged as modulators of cytosolic chaperonin function in eukaryotes (McLaughlin et al., 2002; Lacefield and Solomon, 2003; Martín-Benito et al., 2004; Lukov et al., 2006; Stirling et al., 2006). We now report in vitro and in vivo studies demonstrating that the PhLP2 subgroup also modulates CCT function. Our data suggest that PhLP2 exerts similar effects on CCT as does PhLP3, in that PhLP2 binds CCT, and when in excess, slows protein folding in a ternary complex with substrates (Figures 1 and 6). This functional mechanism may also extend to PhLP1 proteins, which have recently been shown to form ternary complexes with CCT and Gβ to facilitate heterotrimeric G protein assembly (Lukov et al., 2006). Significantly, this is the first study to characterize a PhLP2 protein with respect to CCT function, confirming that members of all three subgroups of phosducin-like proteins cooperate with the chaperonin. This study also describes loss-of-function alleles of PLP2 for the first time and reveals both cytoskeletal and cell cycle phenotypes consistent with a role in modulating CCT activity in vivo. Together these data contribute to the emerging picture of phosducin-like proteins as bona fide CCT cofactors.
Although phosducin-like proteins have generally been implicated in heterotrimeric G protein signaling (Schroder and Lohse, 1996), here we establish that PLP2 is unlikely to play a critical role in heterotrimeric G protein signaling (Supplemental Figure S1). Instead, the function of Plp2p seems closely aligned with that of CCT; Plp2p binds CCT in yeast and ts-alleles of PLP2 and CCT subunits exhibit similar phenotypes, consistent with a functional cooperation between the CCT chaperonin and Plp2p (Figures 1–7; Ursic et al., 1994; Vinh and Drubin, 1994; Siegers et al., 1999; Camasses et al., 2003). Importantly, and similar to CCT (Ursic et al., 1994; Vinh and Drubin, 1994), we conclusively demonstrate that PLP2 is essential for viability in S. cerevisiae (Figure 1).
Cytoskeletal Phenotypes in plp2 Loss-of-Function Cells
The reason for the essential nature of PLP2 is not entirely clear, although we establish cytoskeletal phenotypes as a profound cellular defect of cells lacking functional Plp2p. Cells carrying plp2–ts alleles are sensitive to the drugs latrunculin and benomyl, which disrupt actin and tubulin filaments, respectively (Figure 3). The plp2–ts strains also exhibit a host of other phenotypes, all of which can be indirect indicators of cytoskeletal defects (Figure 4, Supplemental Tables S3–S6, and Supplemental Figure S2). Moreover, the mitotic spindles of plp2-1 and plp2-2 cells become misoriented with respect to the mother-daughter axis when the cells are cultured at high temperature (Figure 4 and Supplemental Table S7). The plp2-ts strains also show weakened polarization of actin filaments and a nearly complete loss of actin cables (Figure 5 and Supplemental Table S8). Together, these data support a role for Plp2p in actin and tubulin function, and they are consistent with previous findings that a reduction in PLP2 expression by using a doxycycline-repressible promoter allele leads to synthetic growth defects in BNI1 or ARP2 mutant backgrounds (Mnaimneh et al., 2004; Davierwala et al., 2005). Bni1p (formin) and the actin-related Arp2p protein are involved in the formation of actin cables and actin patches, respectively (for review, see Evangelista et al., 2003).
The interaction between Plp2p and CCT (Figure 1) strongly supports a role for Plp2p in the production of functional actin and tubulin. Interestingly, our data also suggest that actin-based functions may be more sensitive to Plp2p disruption than are tubulin-based functions. This may indicate that the cellular demand for properly folded actin in the budding cell is higher than it is for tubulin, or that actin-based phenotypes manifest before tubulin-specific defects. Our studies provide unequivocal evidence that both actin and tubulin functions are compromised when Plp2p function is perturbed. Furthermore, the effects of Plp2p are likely mediated via a direct functional interaction with CCT alone, or with CCT-substrate protein complexes (Figures 1 and 6).
Cell Cycle Phenotypes in plp2 Loss-of-Function Cells
Although our characterization of the plp2-ts alleles showed many cytoskeletal phenotypes consistent with a functional interaction with CCT, an unbiased high-copy suppression screen revealed a link to the cell cycle, especially the progression out of G1 phase into S phase. When we examined cell cycle progression by DNA content in plp2-1 cells, we found a delay in DNA replication (Figure 7). Moreover, we observed an excess of unbudded cells when the mutant strains were cultured at high temperature (Supplemental Table S9). Although it is possible that some of these defects relate to actin dysfunction, which can delay budding (as we observed in Supplemental Figure S5), it is likely that Plp2p also affects one or more noncytoskeletal, cell cycle-related CCT substrate(s), as we elaborate below.
Other groups have shown that CCT has an important role in cell cycle progression, because of its effect on the biogenesis of the anaphase promoting complex (APC) regulators Cdc20p and Cdh1p as well as the protein phosphatase subunit Cdc55p, any one of which could be regulated by Plp2p (Camasses et al., 2003; Siegers et al., 2003). Because these particular CCT substrates contain WD-repeats, a role for Plp2p seems plausible in light of the known role for other phosducin-like proteins in WD-repeat folding/assembly by CCT (Lukov et al., 2005, 2006). For example, impaired CCT function leads to precocious entry into S phase because of loss of Cdh1-APC activity, which normally inhibits entry into S phase (Harper et al., 2002; Camasses et al., 2003). If Plp2p were acting to negatively regulate CCT-mediated Cdh1p-APC assembly, then plp2-1 alleles may accumulate excess Cdh1p-APC, thus slowing entry into S phase.
An alternative connection supported by the literature is one between CCT and type 2A protein phosphatases (PP2As). CCT is known to assist the folding of the yeast PP2A regulator Cdc55p, and physical interactions have been reported between CCT and the yeast phosphatase components Sit4p, Pph21p, Pph22p, Pph3p, and Tap42p (Ho et al., 2002; Siegers et al., 2003; Gavin et al., 2006). The finding that PAM1, which bypasses a loss of PP2A activity (Hu and Ronne, 1994), can weakly suppress plp2-1 and plp2-2 alleles (Figure 7A; data not shown) is consistent with a role for Plp2p and CCT in the folding of PP2A components. Indeed, all the suppressors we identified were also identified in a screen for suppression of lethality at the G1/S phase transition in a strain lacking the phosphatase SIT4 and the phosphatase regulator HAL3 (Muñoz et al., 2003). Intriguingly, the toxicity associated with overexpression of SIT4 and SAP155 is suppressed by increased CCT6 copy number in a manner that is not understood (Kabir et al., 2005).
These putative connections of PLP2 function to the cell cycle would help to explain the diverse and weak G1-related suppressors we identified as well as the polarization, cytoskeletal, and cell cycle defects resulting from plp2 mutations. Also of importance is the finding that, in mammalian cells, CCT itself was shown to be up-regulated at the G1/S phase transition and CCT depletion caused arrest of cells at the G1/S phase transition (Yokota et al., 1999; Grantham et al., 2006). These observations suggest an increased folding requirement for some CCT substrates at this cell cycle stage, consistent with our findings.
CONCLUSIONS
Given the literature and the data presented here, we propose a model wherein Plp2p functions to regulate the folding of several CCT substrates that together control cytoskeletal morphogenesis and cell cycle progression. Based on the phenotypes we observed in plp2–ts alleles, candidates for these critical substrates include actin and actin-related proteins, tubulin, Cdc20p, Cdc55p, Cdh1p, and/or as-yet-unconfirmed CCT substrate(s) such as Sit4p. In this model, the additive effects of their altered folding by CCT contribute to the complex phenotypes and eventual lethality associated with loss of PLP2 function. Alternatively, there could be one critical substrate whose biogenesis wholly depends on Plp2p, although we favor the former option because of the PLP2 mutant phenotypes. Actin seems to be particularly affected by PLP2 mutations, suggesting an important role in controlling actin biogenesis and/or the function of an actin regulator such as Bni1p/Arp2p. The conserved nature of PhLP-CCT cooperation and the diversity of substrates likely to be impacted by PhLPs reveal the importance of understanding PhLPs to gain a complete picture of cytosolic chaperonin function in eukaryotes.
ACKNOWLEDGMENTS
We thank Dr. Joaquín Ariño for plasmids and Drs. Doris Ursic (University of Wisconsin, Madison, WI), Timothy Huffaker (Cornell University, Ithaca, NY) and Christopher Beh (Simon Fraser University, Burnaby, British Columbia, Canada) for strains. We also thank Andrea Feigl for technical assistance and Gabriel Alfaro and Chris Beh for technical advice and helpful comments on the manuscript. This research was funded by the Canadian Institutes of Health Research (CIHR; grant BMA121093 to M.R.L.). This work was supported by scholar awards from the Michael Smith Foundation for Health Research (MSFHR) and the CIHR to M.R.L., scholarships from the Natural Sciences and Engineering Research Council of Canada and MSFHR to P.C.S., and a Human Frontier Science Program Long-Term Fellowship to M.S.
Abbreviations used:
- CCT
Chaperonin Containing Tcp1
- DAPI
4′,6-diamidino-2-phenylindole
- FACS
fluorescence activated cell sorting
- PFD
prefoldin
- PhLP
phosducin-like protein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-01-0069) on April 11, 2007.
REFERENCES
- Adams A., Gottschling D. E., Kaiser C. A., Stearns T. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. [Google Scholar]
- Amberg D. C., Burke D. J., Strathern J. N. 2005 ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2005. Methods in Yeast Genetics. [Google Scholar]
- Ayscough K. R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D. G. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauw M., Knol J. C., Kortholt A., Roelofs J., Ruchira , Postma M., Visser A. J., van Haastert P. J. Phosducin-like proteins in Dictyostelium discoideum: implications for the phosducin family of proteins. EMBO J. 2003;22:5047–5057. doi: 10.1093/emboj/cdg508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camasses A., Bogdanova A., Shevchenko A., Zachariae W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol. Cell. 2003;12:87–100. doi: 10.1016/s1097-2765(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5′ ends encode secreted with intracellular forms of yeast invertase. Cell. 1982;28:145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Cowan N. J. Mammalian cytosolic chaperonin. Methods Enzymol. 1998;290:230–241. doi: 10.1016/s0076-6879(98)90022-2. [DOI] [PubMed] [Google Scholar]
- Davierwala A. P., et al. The synthetic genetic interaction spectrum of essential genes. Nat. Genet. 2005;37:1147–1152. doi: 10.1038/ng1640. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Jones H. D., Wertman K. F. Actin structure and function: roles in mitochondrial organization and morphogenesis in budding yeast and identification of the phalloidin-binding site. Mol. Biol. Cell. 1993;4:277–294. doi: 10.1091/mbc.4.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein C. B., Cross F. R. CLB 5, a novel B cyclin from budding yeast with a role in S phase. Genes Dev. 1992;6:1695–1706. doi: 10.1101/gad.6.9.1695. [DOI] [PubMed] [Google Scholar]
- Evangelista M., Zigmond S., Boone C. Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 2003;116:2603–2611. doi: 10.1242/jcs.00611. [DOI] [PubMed] [Google Scholar]
- Flanary P. L., DiBello P. R., Estrada P., Dohlman H. G. Functional analysis of Plp1 and Plp2, two homologues of phosducin in yeast. J. Biol. Chem. 2000;275:18462–18469. doi: 10.1074/jbc.M002163200. [DOI] [PubMed] [Google Scholar]
- Gavin A. C., et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 2006;440:631–636. doi: 10.1038/nature04532. [DOI] [PubMed] [Google Scholar]
- Gao Y., Thomas J. O., Chow R. L., Lee G.-H., Cowan N. J. A cytoplasmic chaperonin that catalyzes β-actin folding. Cell. 1992;69:1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- Gaudet R., Bohm A., Sigler P. B. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- Geissler S., Siegers K., Schiebel E. A novel protein complex promoting formation of functional α- and γ-tubulin. EMBO J. 1998;17:952–966. doi: 10.1093/emboj/17.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J., Brackley K. I., Willison K. R. Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp. Cell. Res. 2006;312:2309–2324. doi: 10.1016/j.yexcr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Burton J. L., Solomon M. J. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 2002;16:2179–2206. doi: 10.1101/gad.1013102. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Ho Y., et al. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature. 2002;415:180–183. doi: 10.1038/415180a. [DOI] [PubMed] [Google Scholar]
- Hu G. Z., Ronne H. Overexpression of yeast PAM1 gene permits survival without protein phosphatase 2A and induces a filamentous phenotype. J. Biol. Chem. 1994;269:3429–3435. [PubMed] [Google Scholar]
- Kabir M. A., et al. Physiological effects of unassembled chaperonin Cct subunits in the yeast Saccharomyces cerevisiae. Yeast. 2005;22:219–239. doi: 10.1002/yea.1210. [DOI] [PubMed] [Google Scholar]
- Lacefield S., Solomon F. A novel step in beta-tubulin folding is important for heterodimer formation in Saccharomyces cerevisiae. Genetics. 2003;165:531–541. doi: 10.1093/genetics/165.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux M. R. Analysis of eukaryotic molecular chaperone complexes involved in actin folding. Methods Mol. Biol. 2000;140:195–206. doi: 10.1385/1-59259-061-6:195. [DOI] [PubMed] [Google Scholar]
- Lopez P., Yaman R., Lopez-Fernandez L. A., Vidal F., Puel D., Clertant P., Cuzin F., Rassoulzadegan M. A novel germ line-specific gene of the phosducin-like protein (PhLP) family: a meiotic function conserved from yeast to mice. J. Biol. Chem. 2003;278:1751–1757. doi: 10.1074/jbc.M207434200. [DOI] [PubMed] [Google Scholar]
- Lukov G. L., Baker C. M., Ludtke P. J., Hu T., Carter M. D., Hackett R. A., Thulin C. D., Willardson B. M. Mechanism of assembly of G protein betagamma subunits by protein kinase CK2-phosphorylated phosducin-like protein and the cytosolic chaperonin complex. J. Biol. Chem. 2006;281:22261–22274. doi: 10.1074/jbc.M601590200. [DOI] [PubMed] [Google Scholar]
- Lukov G. L., Hu T., McLaughlin J. N., Hamm H. E., Willardson B. M. Phosducin-like protein acts as a molecular chaperone for G protein betagamma dimer assembly. EMBO J. 2005;24:1965–1975. doi: 10.1038/sj.emboj.7600673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin V. F., Stirling P. C., Gomez-Reino J., Mwenifumbo J. C., Obst J. M., Valpuesta J. M., Leroux M. R. Molecular clamp mechanism of substrate binding by hydrophobic coiled-coil residues of the archaeal chaperone prefoldin. Proc. Natl. Acad. Sci. USA. 2004;101:4367–4372. doi: 10.1073/pnas.0306276101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Benito J., Bertrand S., Hu T., Ludtke P. J., McLaughlin J. N., Willardson B. M., Carrascosa J. L., Valpuesta J. M. Structure of the complex between the cytosolic chaperonin CCT and phosducin-like protein. Proc. Natl. Acad. Sci. USA. 2004;101:17410–17415. doi: 10.1073/pnas.0405070101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. N., Thulin C. D., Hart S. J., Resing K. A., Ahn N. G., Willardson B. M. Regulatory interaction of phosducin-like protein with the cytosolic chaperonin complex. Proc. Natl. Acad. Sci. USA. 2002;99:7962–7967. doi: 10.1073/pnas.112075699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan J. N., Sia R. A., Lew D. J. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol. 1998;142:1487–1499. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R., Batelier G., Soulie S., Williams R. C. Cytoplasmic chaperonin containing TCP-1, structural and functional characterization. Biochemistry. 1997;36:5817–5826. doi: 10.1021/bi962830o. [DOI] [PubMed] [Google Scholar]
- Mnaimneh S., et al. Exploration of essential gene functions via titratable promoter alleles. Cell. 2004;118:31–44. doi: 10.1016/j.cell.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Muñoz I., Simon E., Casals N., Clotet J., Arino J. Identification of multicopy suppressors of cell cycle arrest at the G1/S transition in Saccharomyces cerevisiae. Yeast. 2003;20:157–169. doi: 10.1002/yea.938. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Matsubayashi Y., Nishida E. An evolutionarily conserved gene required for proper microtubule architecture in Caenorhabditis elegans. Genes Cells. 2004;9:83–93. doi: 10.1111/j.1356-9597.2004.00708.x. [DOI] [PubMed] [Google Scholar]
- Schroder S., Lohse M. J. Inhibition of G-protein betagamma-subunit functions by phosducin-like protein. Proc. Natl. Acad. Sci. USA. 1996;93:2100–2104. doi: 10.1073/pnas.93.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob E., Nasmyth K. CLB5 and CLB6, a new pair of B cyclins involved in DNA replication in Saccharomyces cerevisiae. Genes Dev. 1993;7:1160–1175. doi: 10.1101/gad.7.7a.1160. [DOI] [PubMed] [Google Scholar]
- Siegert R., Leroux M. R., Scheufler C., Hartl F. U., Moarefi I. Structure of the molecular chaperone prefoldin. Unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell. 2000;103:621–632. doi: 10.1016/s0092-8674(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Siegers K., Waldmann T., Leroux M. R., Grein K., Shevchenko A., Schiebel E., Hartl F. U. Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 1999;18:75–84. doi: 10.1093/emboj/18.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegers K., Bolter B., Schwarz J. P., Bottcher U. M., Guha S., Hartl F. U. TRiC/CCT cooperates with different upstream chaperones in the folding of distinct protein classes. EMBO J. 2003;22:5230–5240. doi: 10.1093/emboj/cdg483. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Spiess C., Meyer A. S., Reissmann S., Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 2004;14:598–604. doi: 10.1016/j.tcb.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling P. C., Cuellar J., Alfaro G. A., El Khadali F., Beh C. T., Valpuesta J. M., Melki R., Leroux M. R. PhLP3 modulates CCT-mediated actin and tubulin folding via ternary complexes with substrates. J. Biol. Chem. 2006;281:7012–7021. doi: 10.1074/jbc.M513235200. [DOI] [PubMed] [Google Scholar]
- Theesfeld C. L., Irazoqui J. E., Bloom K., Lew D. J. The role of actin in spindle orientation changes during the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 1999;146:1019–1032. doi: 10.1083/jcb.146.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulasiraman V., Yang C. F., Frydman J. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J. 1999;18:85–95. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursic D., Sedbrook J. C., Himmel K. L., Culbertson M. R. The essential yeast Tcp1 protein affects actin and microtubules. Mol. Biol. Cell. 1994;5:1065–1080. doi: 10.1091/mbc.5.10.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainberg I. E., Lewis S. A., Rommelaere H., Ampe C., Vandekerckhove J., Klein H. L., Cowan N. J. Prefoldin, a chaperone that delivers unfolded proteins to cytosolic chaperonin. Cell. 1998;93:863–873. doi: 10.1016/s0092-8674(00)81446-4. [DOI] [PubMed] [Google Scholar]
- Vinh D. B., Drubin D. G. A yeast TCP-1-like protein is required for actin function in vivo. Proc. Natl. Acad. Sci. USA. 1994;91:9116–9120. doi: 10.1073/pnas.91.19.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. C., Richter B. W., Wilkinson A. S., Burstein E., Rumble J. M., Balliu B., Duckett C. S. VIAF, a conserved inhibitor of apoptosis (IAP)-interacting factor that modulates caspase activation. J. Biol. Chem. 2004;279:51091–51099. doi: 10.1074/jbc.M409623200. [DOI] [PubMed] [Google Scholar]
- Yokota S., Yanagi H., Yura T., Kubota H. Cytosolic chaperonin is up-regulated during cell growth. Preferential expression and binding to tubulin at G(1)/S transition through early S phase. J. Biol. Chem. 1999;274:37070–37078. doi: 10.1074/jbc.274.52.37070. [DOI] [PubMed] [Google Scholar]
- Zachariae W., Shevchenko A., Andrews P. D., Ciosk R., Galova M., Stark M. J., Mann M., Nasmyth K. Mass spectrometric analysis of the anaphase-promoting complex from yeast: identification of a subunit related to cullins. Science. 1998;279:1216–1219. doi: 10.1126/science.279.5354.1216. [DOI] [PubMed] [Google Scholar]