Abstract
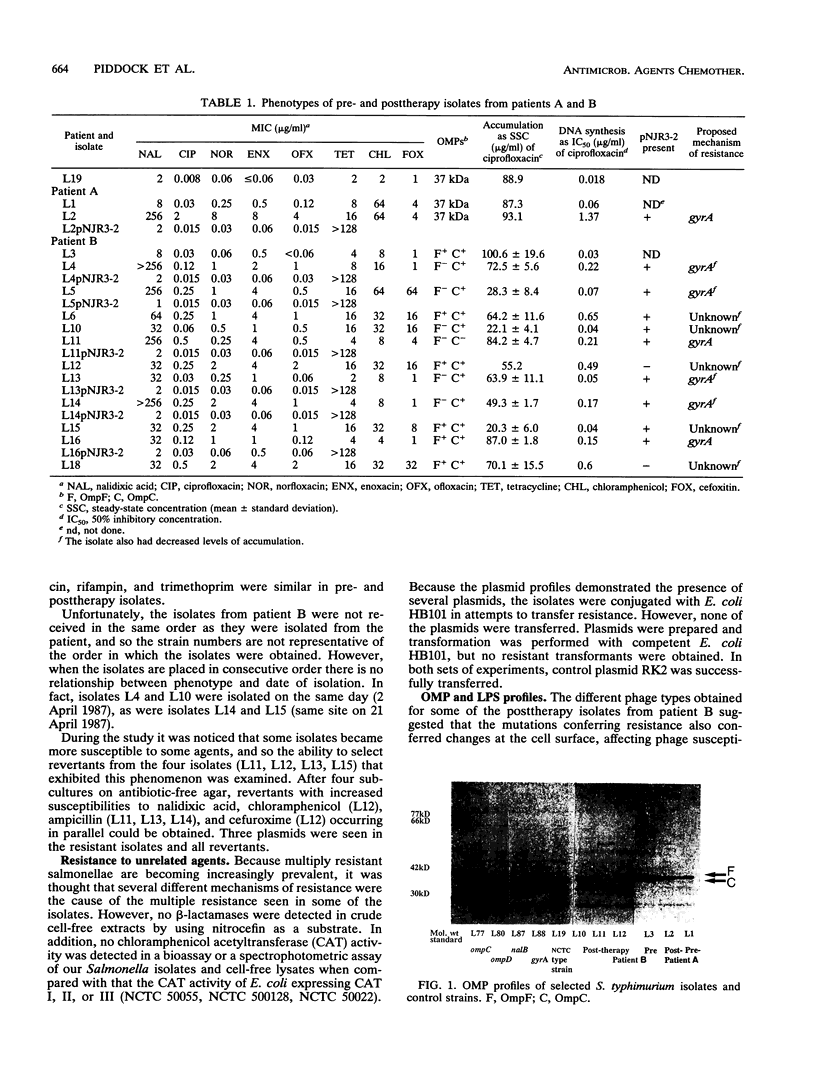
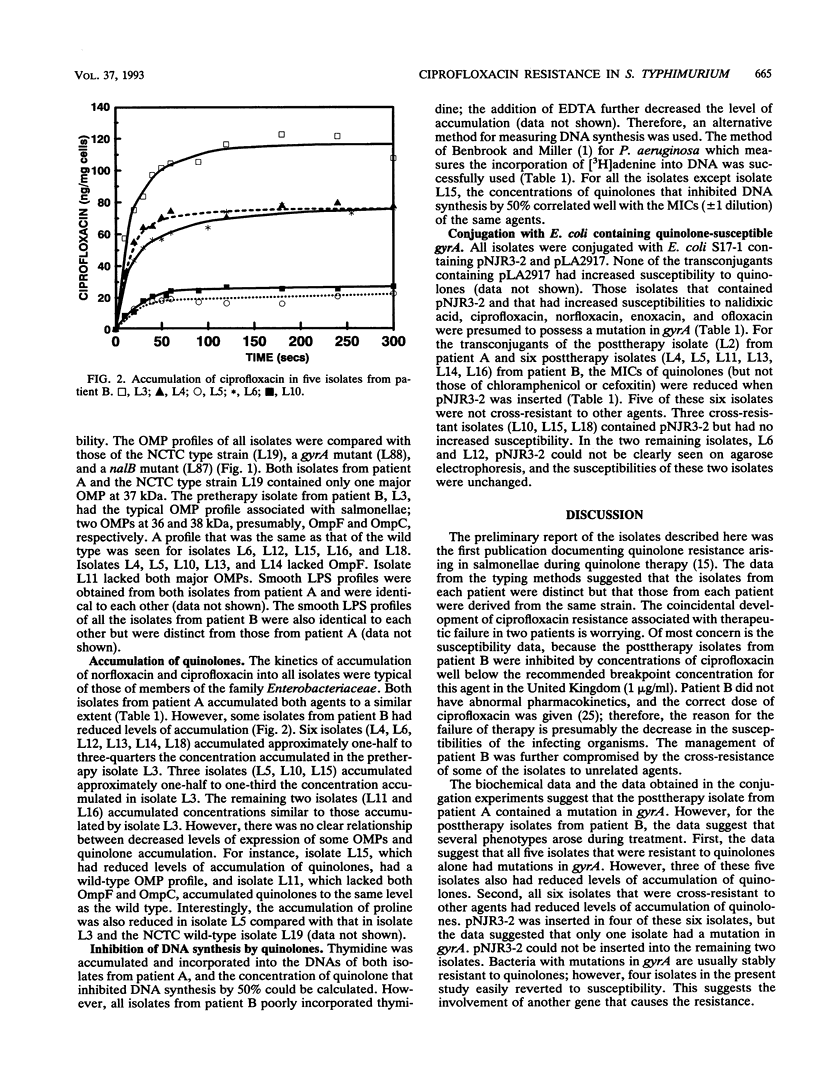
Two patients (patients A and B) infected with Salmonella typhimurium failed ciprofloxacin therapy, and the posttherapy isolates had reduced susceptibilities to quinolones; 6 of 11 isolates from patient B were also cross-resistant to chemically unrelated agents. No transferable resistance, chloramphenicol-acetylating enzymes, or beta-lactamases were detected. For 13 of 14 isolates, the concentrations of ciprofloxacin that inhibited DNA synthesis by 50% were similar to the MICs, suggesting a mutation in gyrA. Insertion of pNJR3-2 (gyrA) in the posttherapy isolate from patient A and 5 of 11 of the posttherapy isolates from patient B resulted in lower quinolone MICs, also suggesting that resistance was due to a mutation in gyrA. Three of the five isolates also had reduced levels of accumulation of quinolones. All six cross-resistant isolates from patient B had reduced levels of accumulation of quinolones, but only one isolate had increased susceptibility when pNJR3-2 was inserted. Despite the lack of OmpF seen in five isolates from patient B, there was no correlation with decreased levels of quinolone accumulation. All isolates had identical smooth lipopolysaccharide profiles. The mechanism of apparently reduced accumulation has yet to be determined.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Heritage J., Hawkey P. M. An ultra-rapid method for the study of antibiotic resistance plasmids. J Antimicrob Chemother. 1986 Sep;18(3):421–424. doi: 10.1093/jac/18.3.421. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hof H., Ehrhard I., Tschäpe H. Presence of quinolone resistance in a strain of Salmonella typhimurium. Eur J Clin Microbiol Infect Dis. 1991 Sep;10(9):747–749. doi: 10.1007/BF01972501. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. J., Joseph T. D., Bloodworth L. L., Frost J. A., Chart H., Rowe B. The emergence of ciprofloxacin resistance in Salmonella typhimurium. J Antimicrob Chemother. 1990 Aug;26(2):296–298. doi: 10.1093/jac/26.2.296. [DOI] [PubMed] [Google Scholar]
- Jewes L. A. Antimicrobial therapy of non-typhi salmonella and Shigella infection. J Antimicrob Chemother. 1987 May;19(5):557–560. doi: 10.1093/jac/19.5.557. [DOI] [PubMed] [Google Scholar]
- Lewin C. S., Nandivada L. S., Amyes S. G. Multiresistant Salmonella and fluoroquinolones. J Antimicrob Chemother. 1991 Jan;27(1):147–149. doi: 10.1093/jac/27.1.147. [DOI] [PubMed] [Google Scholar]
- Mortimer P. G., Piddock L. J. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1991 Nov;28(5):639–653. doi: 10.1093/jac/28.5.639. [DOI] [PubMed] [Google Scholar]
- Murray B. E. Resistance of Shigella, Salmonella, and other selected enteric pathogens to antimicrobial agents. Rev Infect Dis. 1986 May-Jun;8 (Suppl 2):S172–S181. doi: 10.1093/clinids/8.supplement_2.s172. [DOI] [PubMed] [Google Scholar]
- Oram M., Fisher L. M. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother. 1991 Feb;35(2):387–389. doi: 10.1128/aac.35.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J. Ciprofloxacin resistance in Salmonella serotypes. J Antimicrob Chemother. 1990 Dec;26(6):853–853. doi: 10.1093/jac/26.6.853. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Hall M. C., Walters R. N. Phenotypic characterization of quinolone-resistant mutants of Enterobacteriaceae selected from wild type, gyrA type and multiply-resistant (marA) type strains. J Antimicrob Chemother. 1991 Aug;28(2):185–198. doi: 10.1093/jac/28.2.185. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Traynor E. A., Wise R. A comparison of the mechanisms of decreased susceptibility of aztreonam-resistant and ceftazidime-resistant Enterobacteriaceae. J Antimicrob Chemother. 1990 Dec;26(6):749–762. doi: 10.1093/jac/26.6.749. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Whale K., Wise R. Quinolone resistance in salmonella: clinical experience. Lancet. 1990 Jun 16;335(8703):1459–1459. doi: 10.1016/0140-6736(90)91484-r. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. Mechanisms of resistance to quinolones and clinical perspectives. J Antimicrob Chemother. 1989 Apr;23(4):475–480. doi: 10.1093/jac/23.4.475. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Zhu M. Mechanism of action of sparfloxacin against and mechanism of resistance in gram-negative and gram-positive bacteria. Antimicrob Agents Chemother. 1991 Nov;35(11):2423–2427. doi: 10.1128/aac.35.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T. M. The treatment of non-typhi salmonellosis. J Antimicrob Chemother. 1992 Jan;29(1):4–8. doi: 10.1093/jac/29.1.4. [DOI] [PubMed] [Google Scholar]
- Robillard N. J. Broad-host-range gyrase A gene probe. Antimicrob Agents Chemother. 1990 Oct;34(10):1889–1894. doi: 10.1128/aac.34.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Uwaydah A. K., Matar I., Chacko K. C., Davidson J. C. The emergence of antimicrobial resistant Salmonella typhi in Qatar: epidemiology and therapeutic implications. Trans R Soc Trop Med Hyg. 1991 Nov-Dec;85(6):790–792. doi: 10.1016/0035-9203(91)90457-a. [DOI] [PubMed] [Google Scholar]
- Wang F., Gu X. J., Zhang M. F., Tai T. Y. Treatment of typhoid fever with ofloxacin. J Antimicrob Chemother. 1989 May;23(5):785–788. doi: 10.1093/jac/23.5.785. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]



