Abstract
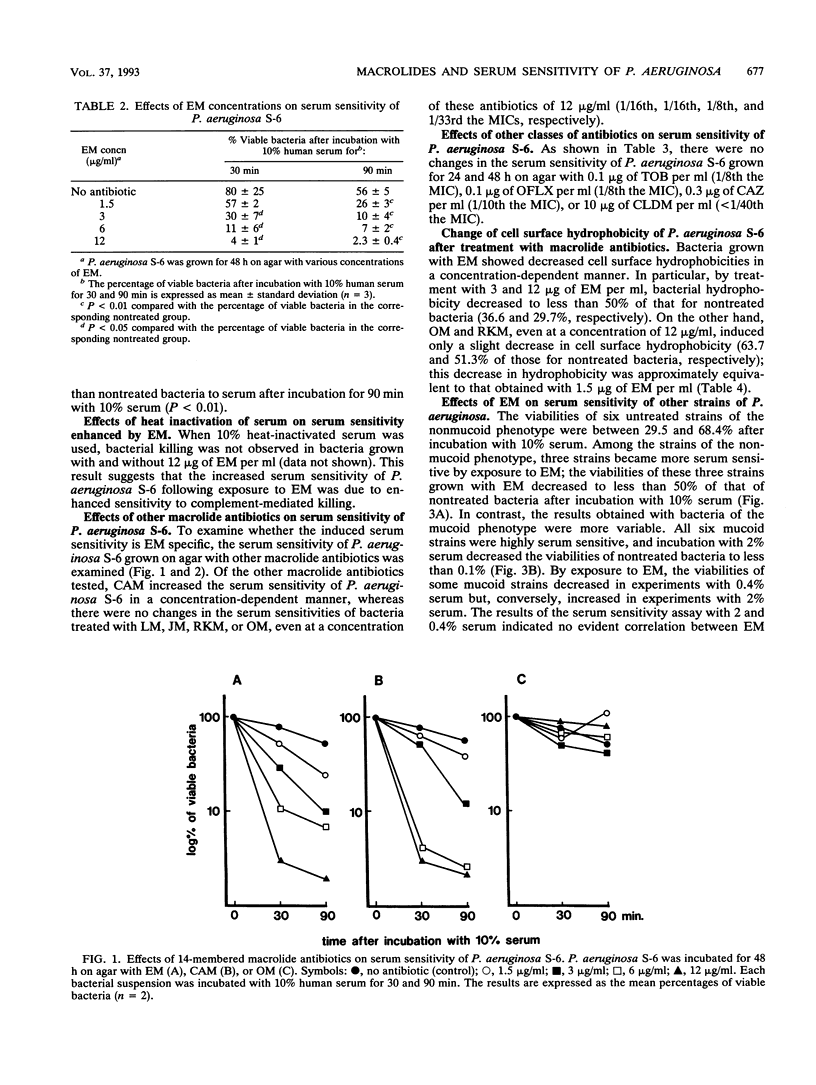
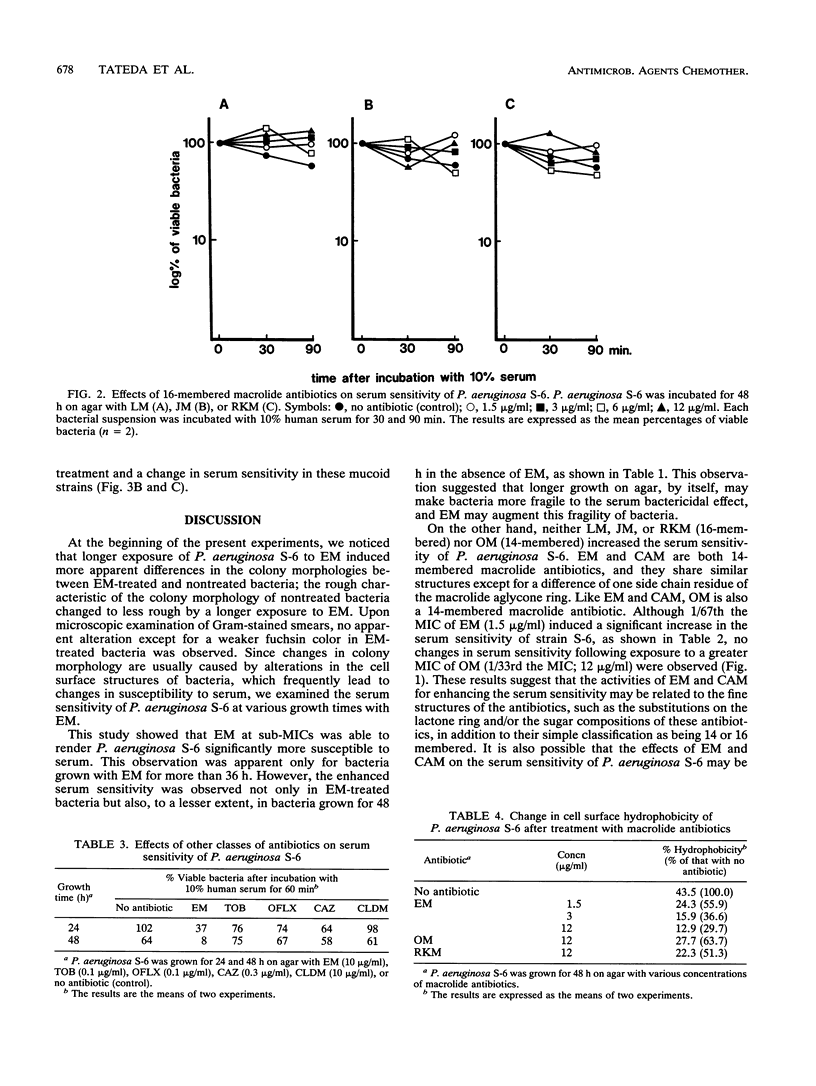
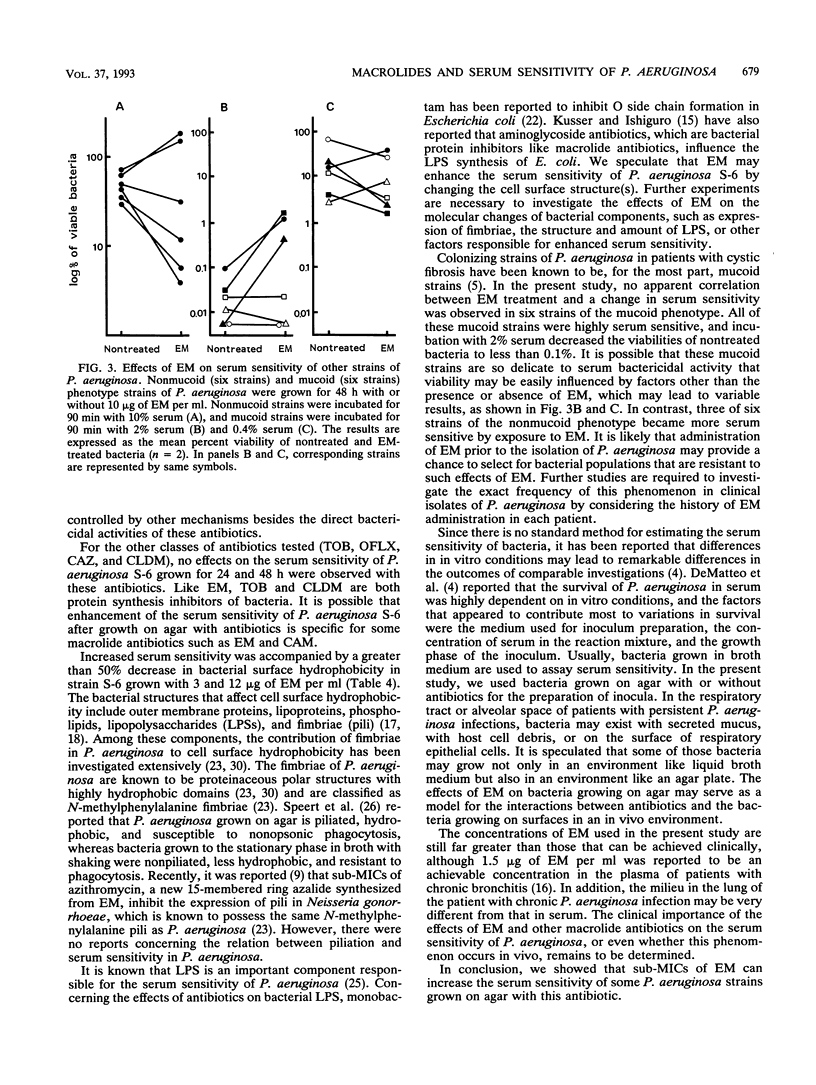
We examined the effects of sub-MICs of erythromycin (EM) and other macrolide antibiotics on the serum sensitivity of Pseudomonas aeruginosa. P. aeruginosa S-6 grown for 36 and 48 h on agar with 10 micrograms of EM per ml (1/10th the MIC) showed significantly increased sensitivity to human serum bactericidal activity compared with those of bacteria grown on agar without EM (P < 0.05). No changes in serum sensitivity were observed in bacteria grown for less than 24 h. This increased sensitivity was apparent even at a concentration of 1.5 micrograms of EM per ml (1/67th the MIC) in bacteria grown for 48 h (P < 0.01). Among the other macrolide antibiotics tested, clarithromycin also enhanced sensitivity to serum, but there were no changes in the sensitivities of bacteria grown on agar with kitasamycin, josamycin, rokitamycin, or oleandomycin even at a concentration of 12 micrograms/ml (1/16th, 1/16th, 1/8th, and 1/33rd the MICs, respectively). P. aeruginosa S-6 grown on agar with subinhibitory concentrations of EM showed decreased cell surface hydrophobicity in a dose-dependent manner, whereas oleandomycin and rokitamycin, even at a concentration of 12 micrograms/ml, induced a slight decrease in hydrophobicity which was approximately equivalent to that of 1.5 micrograms of EM per ml. Among six other strains of the nonmucoid phenotype, three strains became more sensitive to serum by exposure to 10 micrograms of EM per ml for 48 h. In contrast, no evident correlation between EM treatment and a change in serum sensitivity was observed in six strains of the mucoid phenotype, as judged by the results of experiments with both 2 and 0.4% serum. These results show that EM at subinhibitory concentrations enhances the serum sensitivity of some P. aeruginosa strains. Since induced serum sensitivity was accompanied by a decrease in bacterial cell surface hydrophobicity, EM may render P. aeruginosa more serum sensitive by changing the cell surface structure(s) of this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cialdella J. I., Ulrich R. G., Liggett W. F., Adams L. D., Marshall V. P. Effects of trospectomycin on serum sensitivity of Escherichia coli UC 9451. Antimicrob Agents Chemother. 1990 Nov;34(11):2086–2092. doi: 10.1128/aac.34.11.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., Cunningham M. D. Influence of subinhibitory concentrations of cephalosporins on the serum sensitivity of Pseudomonas aeruginosa. J Infect Dis. 1990 Oct;162(4):914–921. doi: 10.1093/infdis/162.4.914. [DOI] [PubMed] [Google Scholar]
- DeMatteo C. S., Hammer M. C., Baltch A. L., Smith R. P., Sutphen N. T., Michelsen P. B. Susceptibility of Pseudomonas aeruginosa to serum bactericidal activity. A comparison of three methods with clinical correlations. J Lab Clin Med. 1981 Oct;98(4):511–518. [PubMed] [Google Scholar]
- Fernandes A. C., Anderson R., Theron A. J., Jooné G., Van Rensburg C. E. Enhancement of human polymorphonuclear leucocyte motility by erythromycin in vitro and in vivo. S Afr Med J. 1984 Aug 4;66(5):173–177. [PubMed] [Google Scholar]
- Fraschini F., Scaglione F., Ferrara F., Marelli O., Braga P. C., Teodori F. Evaluation of the immunostimulating activity of erythromycin in man. Chemotherapy. 1986;32(3):286–290. doi: 10.1159/000238425. [DOI] [PubMed] [Google Scholar]
- Gilligan P. H. Microbiology of airway disease in patients with cystic fibrosis. Clin Microbiol Rev. 1991 Jan;4(1):35–51. doi: 10.1128/cmr.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorby G. L., McGee Z. A. Antimicrobial interference with bacterial mechanisms of pathogenicity: effect of sub-MIC azithromycin on gonococcal piliation and attachment to human epithelial cells. Antimicrob Agents Chemother. 1990 Dec;34(12):2445–2448. doi: 10.1128/aac.34.12.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess K., Kern S., Schiller H. H. Blink reflex in trigeminal sensory neuropathy. Electromyogr Clin Neurophysiol. 1984 Mar-Apr;24(3):185–190. [PubMed] [Google Scholar]
- Hewlett E. L. Selective primary health care: strategies for control of disease in the developing world. XVII. Pertussis and diphtheria. Rev Infect Dis. 1985 May-Jun;7(3):426–433. doi: 10.1093/clinids/7.3.426. [DOI] [PubMed] [Google Scholar]
- Hirakata Y., Kaku M., Mizukane R., Ishida K., Furuya N., Matsumoto T., Tateda K., Yamaguchi K. Potential effects of erythromycin on host defense systems and virulence of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992 Sep;36(9):1922–1927. doi: 10.1128/aac.36.9.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakata Y., Kaku M., Tomono K., Tateda K., Furuya N., Matsumoto T., Araki R., Yamaguchi K. Efficacy of erythromycin lactobionate for treating Pseudomonas aeruginosa bacteremia in mice. Antimicrob Agents Chemother. 1992 Jun;36(6):1198–1203. doi: 10.1128/aac.36.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H., Yamanaka A., Tanimoto S., Tamura M., Chijimatsu Y., Kira S., Izumi T. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest. 1983 Jan;83(1):63–69. doi: 10.1378/chest.83.1.63. [DOI] [PubMed] [Google Scholar]
- Kita E., Sawaki M., Nishikawa F., Mikasa K., Yagyu Y., Takeuchi S., Yasui K., Narita N., Kashiba S. Enhanced interleukin production after long-term administration of erythromycin stearate. Pharmacology. 1990;41(4):177–183. doi: 10.1159/000138716. [DOI] [PubMed] [Google Scholar]
- Kita E., Sawaki M., Oku D., Hamuro A., Mikasa K., Konishi M., Emoto M., Takeuchi S., Narita N., Kashiba S. Suppression of virulence factors of Pseudomonas aeruginosa by erythromycin. J Antimicrob Chemother. 1991 Mar;27(3):273–284. doi: 10.1093/jac/27.3.273. [DOI] [PubMed] [Google Scholar]
- Kusser W. C., Ishiguro E. E. Effects of aminoglycosides and spectinomycin on the synthesis and release of lipopolysaccharide by Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1247–1250. doi: 10.1128/aac.32.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin G. E., Davis P. R., Rutland J., Berend N. Plasma and sputum erythromycin concentrations in chronic bronchitis. Thorax. 1980 Jun;35(6):441–445. doi: 10.1136/thx.35.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes N., Léonard A. J., Rouxhet P. G. On the relations between the elemental surface composition of yeasts and bacteria and their charge and hydrophobicity. Biochim Biophys Acta. 1988 Nov 22;945(2):324–334. doi: 10.1016/0005-2736(88)90495-6. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The role of Pseudomonas aeruginosa in infections. J Antimicrob Chemother. 1983 May;11 (Suppl B):1–13. doi: 10.1093/jac/11.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Overbeek B. P., Schellekens J. F., Lippe W., Dekker B. A., Verhoef J. Carumonam enhances reactivity of Escherichia coli with mono- and polyclonal antisera to rough Escherichia coli J5. J Clin Microbiol. 1987 Jun;25(6):1009–1013. doi: 10.1128/jcm.25.6.1009-1013.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranchych W., Sastry P. A., Frost L. S., Carpenter M., Armstrong G. D., Watts T. H. Biochemical studies on pili isolated from Pseudomonas aeruginosa strain PAO. Can J Microbiol. 1979 Oct;25(10):1175–1181. doi: 10.1139/m79-182. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Loh B. A., Cabral D. A., Salit I. E. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun. 1986 Jul;53(1):207–212. doi: 10.1128/iai.53.1.207-212.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Gaunt H., Unger F. M. Effect of subinhibitory concentrations of mecillinam on the serum susceptibility of Escherichia coli strains. Antimicrob Agents Chemother. 1981 May;19(5):786–788. doi: 10.1128/aac.19.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veringa E., Box A., Rozenberg-Arska M., Verhoef J. Monobactam antibiotics in subinhibitory concentrations enhance opsonophagocytosis and serum bacteriolysis in certain Escherichia coli strains. Drugs Exp Clin Res. 1988;14(1):1–8. [PubMed] [Google Scholar]
- Watts T. H., Sastry P. A., Hodges R. S., Paranchych W. Mapping of the antigenic determinants of Pseudomonas aeruginosa PAK polar pili. Infect Immun. 1983 Oct;42(1):113–121. doi: 10.1128/iai.42.1.113-121.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]


