Abstract
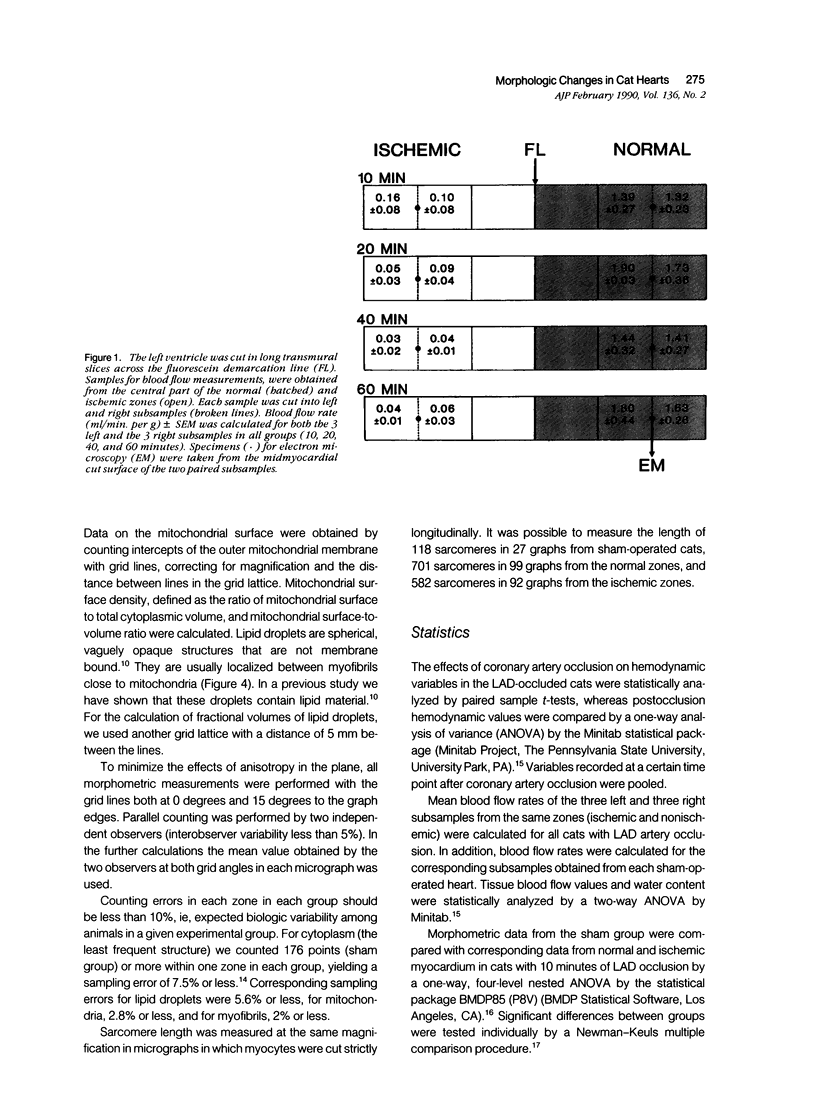
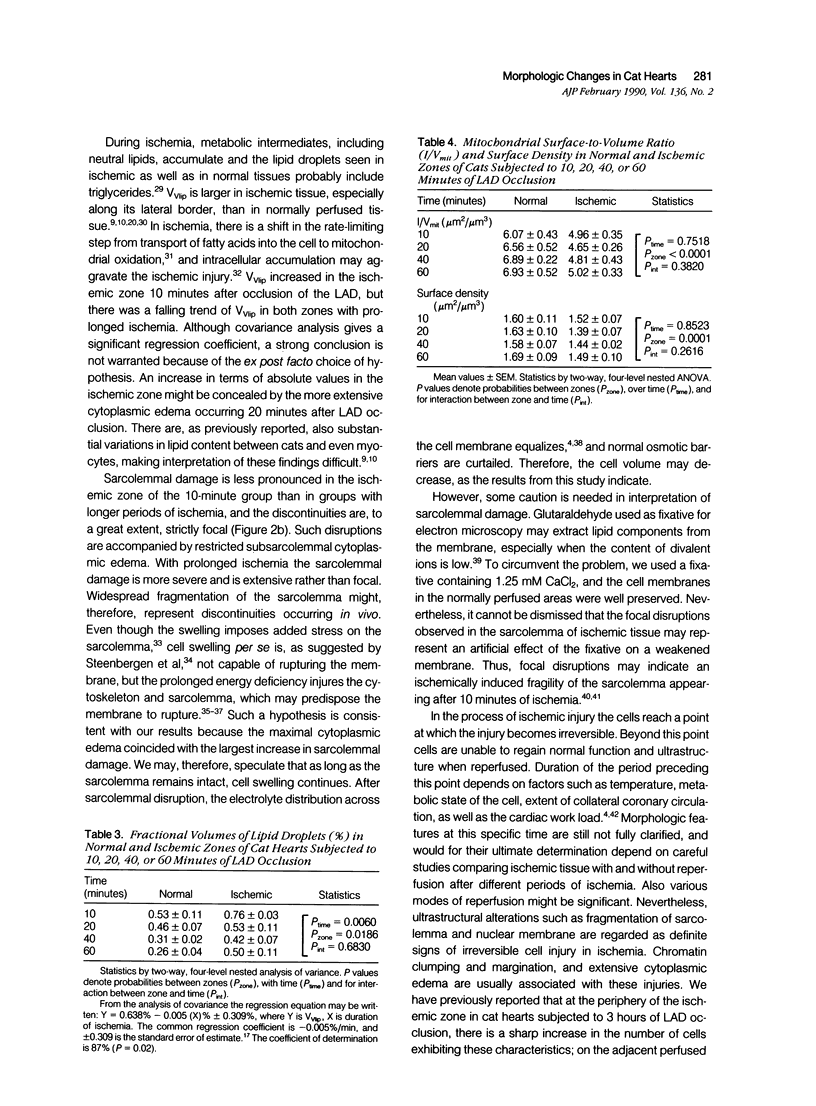
The left descending coronary artery (LAD) was occluded in 16 open-chest cats for 10, 20, 40, or 60 minutes (four cats in each group). In addition, four sham-operated cats served as controls. Specimens for electron microscopy were obtained from the normal and ischemic zones, guided by in vivo injection of fluorescein, and verified by blood flow measurements with microspheres. The ultrastructure of 2,400 heart muscle cells and nuclei was studied. Fractional volumes of main cell components, mitochondrial surface density, and mitochondrial surface: volume ratio were calculated in 480 micrographs. After 10 minutes of ischemia we observed signs of sarcolemmal fragility, mitochondrial swelling, and lipid droplet accumulation. After 20 minutes of ischemia sarcolemmal fragmentation, chromatin clumping or margination and a maximal cytoplasmic edema were evident. The fractional volume of mitochondria was equally increased in ischemic zones of all groups. In both normal and ischemic zones there was a tendency toward smaller fractional volumes of lipid droplets during ischemia. In the normal zone there was mild cytoplasmic edema and slight mitochondrial swelling 10 minutes after occlusion as compared with the sham group. The present study demonstrates that a large proportion of cardiac myocytes undergoes severe damage within 20 minutes of coronary occlusion.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banka V. S., Bodenheimer M. M., Ramanathan K. B., Hermann G. A., Helfant R. H. Progressive transmural electrographic, myocardial potassium ion/sodium ion ratio and ultrastructural changes as a function of time after acute coronary occlusion. Am J Cardiol. 1978 Sep;42(3):429–443. doi: 10.1016/0002-9149(78)90938-4. [DOI] [PubMed] [Google Scholar]
- Corr P. B., Gross R. W., Sobel B. E. Amphipathic metabolites and membrane dysfunction in ischemic myocardium. Circ Res. 1984 Aug;55(2):135–154. doi: 10.1161/01.res.55.2.135. [DOI] [PubMed] [Google Scholar]
- Fukuhara T. Histochemical, ultrastructural and cytochemical study of reperfusion effect on ischemic myocardial injury. Jpn Circ J. 1985 Apr;49(4):432–445. doi: 10.1253/jcj.49.432. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Vander Heide R. S. Cytoskeletal lesions in anoxic myocardial injury. A conventional and high-voltage electron-microscopic and immunofluorescence study. Am J Pathol. 1987 Nov;129(2):327–344. [PMC free article] [PubMed] [Google Scholar]
- Greve G., Rotevatn S., Grong K., Stangeland L. Cellular morphometric changes in cat hearts subjected to three hours of regional ischaemia. Virchows Arch A Pathol Anat Histopathol. 1988;412(3):205–213. doi: 10.1007/BF00737144. [DOI] [PubMed] [Google Scholar]
- Greve G., Rotevatn S., Stangeland L. Morphological changes across the border zone of cat hearts subjected to regional ischaemia. Virchows Arch A Pathol Anat Histopathol. 1989;415(4):323–333. doi: 10.1007/BF00718635. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A. Lethal myocardial ischemic injury. Am J Pathol. 1981 Feb;102(2):241–255. [PMC free article] [PubMed] [Google Scholar]
- Jennings R. B., Reimer K. A., Steenbergen C. Myocardial ischemia revisited. The osmolar load, membrane damage, and reperfusion. J Mol Cell Cardiol. 1986 Aug;18(8):769–780. doi: 10.1016/s0022-2828(86)80952-x. [DOI] [PubMed] [Google Scholar]
- Jodalen H., Stangeland L., Grong K., Vik-Mo H., Lekven J. Lipid accumulation in the myocardium during acute regional ischaemia in cats. J Mol Cell Cardiol. 1985 Oct;17(10):973–980. doi: 10.1016/s0022-2828(85)80077-8. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Braunwald E. Observations on experimental myocardial ischaemia. Cardiovasc Res. 1980 Jul;14(7):371–395. doi: 10.1093/cvr/14.7.371. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Fishbein M. C., Maclean D., Braunwald E., Maroko P. R. Effect of hyaluronidase during the early phase of acute myocardial ischemia: an ultrastructural and morphometric analysis. Am J Cardiol. 1977 Jul;40(1):43–49. doi: 10.1016/0002-9149(77)90098-4. [DOI] [PubMed] [Google Scholar]
- McCallister L. P., Trapukdi S., Neely J. R. Morphometric observations on the effects of ischemia in the isolated perfused rat heart. J Mol Cell Cardiol. 1979 Jul;11(7):619–630. doi: 10.1016/0022-2828(79)90376-6. [DOI] [PubMed] [Google Scholar]
- Spinale F. G., Schulte B. A., Crawford F. A. Demonstration of early ischemic injury in porcine right ventricular myocardium. Am J Pathol. 1989 Mar;134(3):693–704. [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C., Hill M. L., Jennings R. B. Cytoskeletal damage during myocardial ischemia: changes in vinculin immunofluorescence staining during total in vitro ischemia in canine heart. Circ Res. 1987 Apr;60(4):478–486. doi: 10.1161/01.res.60.4.478. [DOI] [PubMed] [Google Scholar]
- Steenbergen C., Hill M. L., Jennings R. B. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985 Dec;57(6):864–875. doi: 10.1161/01.res.57.6.864. [DOI] [PubMed] [Google Scholar]
- Vander Heide R. S., Ganote C. E. Increased myocyte fragility following anoxic injury. J Mol Cell Cardiol. 1987 Nov;19(11):1085–1103. doi: 10.1016/s0022-2828(87)80353-x. [DOI] [PubMed] [Google Scholar]
- Vasdev S. C., Biro G. P., Narbaitz R., Kako K. J. Membrane changes induced by early myocardial ischemia in the dog. Can J Biochem. 1980 Oct;58(10):1112–1119. doi: 10.1139/o80-149. [DOI] [PubMed] [Google Scholar]
- Ward B., Harris P. Incorporation and distribution of 3H oleic acid in the isolated, perfused guinea-pig heart made hypoxic. J Mol Cell Cardiol. 1984 Aug;16(8):765–770. doi: 10.1016/s0022-2828(84)80659-8. [DOI] [PubMed] [Google Scholar]






