Abstract
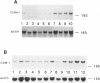
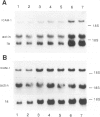
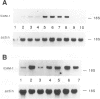
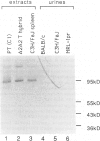
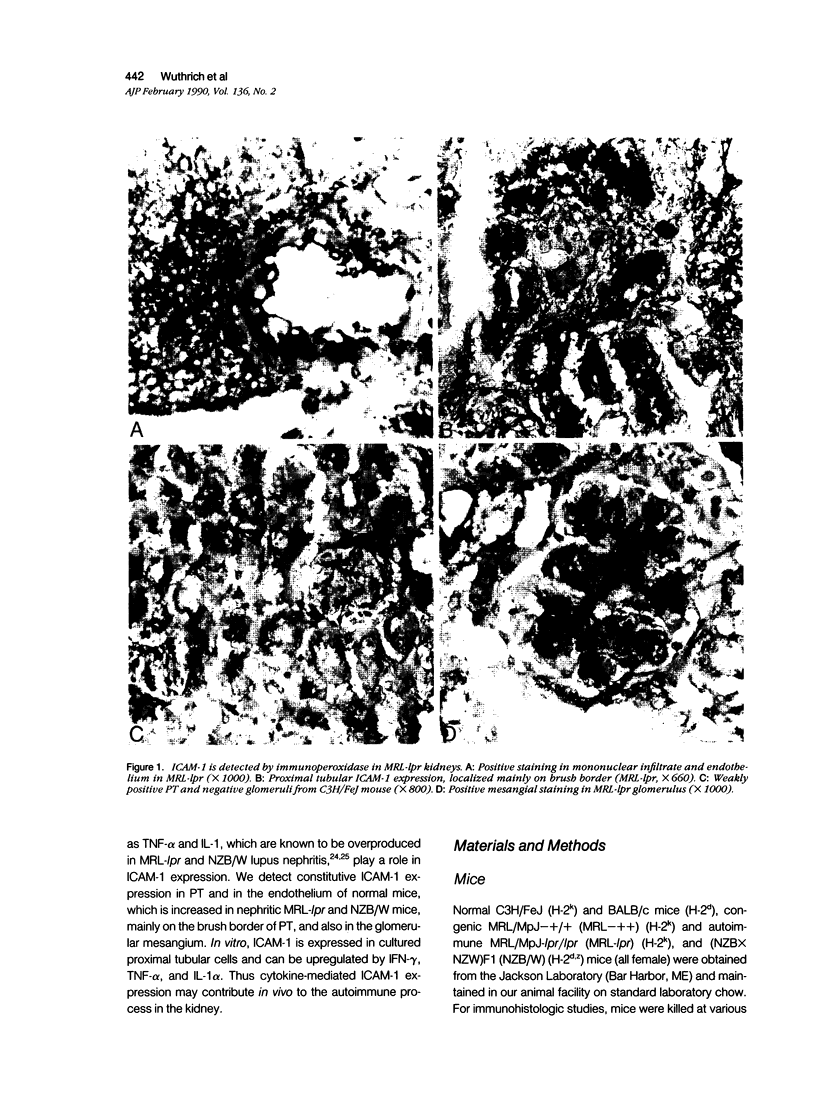
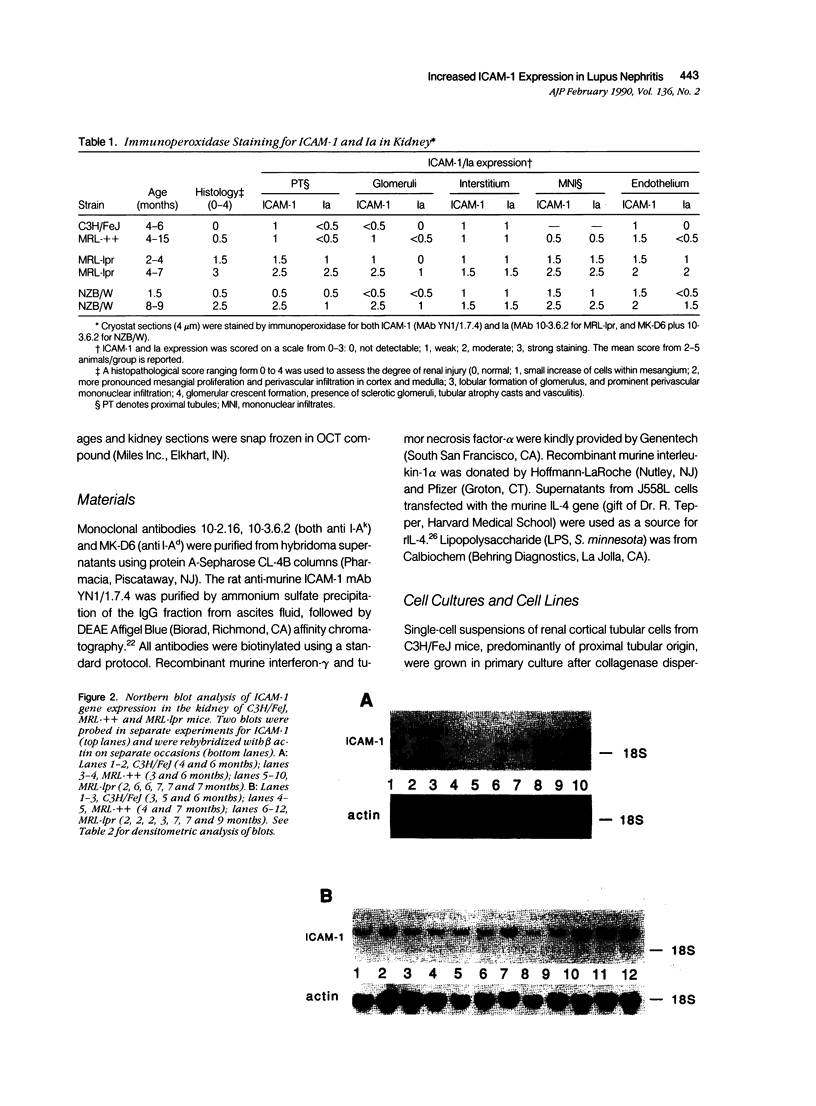
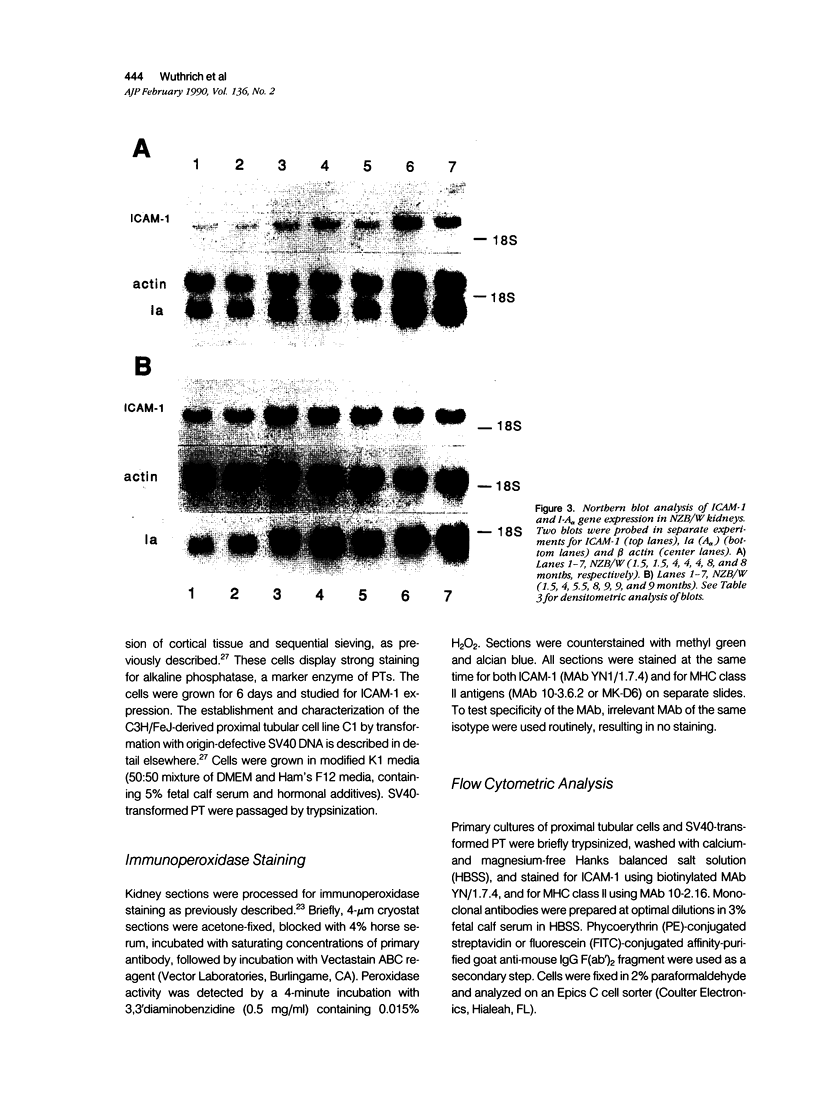
Intercellular adhesion molecule-1 (ICAM-1) is a cell-surface protein regulating interactions among immune cells. To determine whether altered expression of ICAM-1 occurs in autoimmune lupus nephritis, we studied ICAM-1 expression in kidneys of normal and autoimmune MRL-lpr and (NZBX NZW)F1 (NZB/W) mice. By immunoperoxidase staining, ICAM-1 is constitutively expressed at low levels in proximal tubules (PT), endothelium and interstitial cells in normal C3H/FeJ mice. In nephritic MRL-lpr and NZB/W kidneys, staining for ICAM-1 is increased in the PT, particularly in the brush border, and is prominent in the glomerular mesangium and the endothelium of large vessels. By Western blot analysis, ICAM-1 is not detected in the urine of normal BALB/c and C3H/FeJ or autoimmune MRL-lpr. By Northern blot analysis, nephritic MRL-lpr and NZB/W have a two- to fivefold increase in steady state levels of ICAM-1 transcripts in the kidney as compared with normal or prenephritic mice. This is paralleled by an increase in MHC class II transcripts. In cultured PT cells, ICAM-1 is expressed at basal levels in PT and is increased by the cytokines interferon-gamma, IL-1 alpha, and TNF-alpha. Thus cytokine-mediated upregulation of ICAM-1 in lupus nephritis may promote interaction of immune cells with renal tissue. The predominant apical expression of ICAM-1 opposite to the basolateral Ia expression suggests a novel role for this adhesion molecule in PT.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A., Choi E., McIntyre K. R., Leeman S. A., McKean D. J., Seidman J. G., Glimcher L. H. DNA-mediated transfer of major histocompatibility class II I-Ab and I-Abm12 genes into B lymphoma cells: molecular and functional analysis of introduced antigens. J Immunol. 1985 Aug;135(2):1456–1464. [PubMed] [Google Scholar]
- Boswell J. M., Yui M. A., Burt D. W., Kelley V. E. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988 Nov 1;141(9):3050–3054. [PubMed] [Google Scholar]
- Boswell J. M., Yui M. A., Endres S., Burt D. W., Kelley V. E. Novel and enhanced IL-1 gene expression in autoimmune mice with lupus. J Immunol. 1988 Jul 1;141(1):118–124. [PubMed] [Google Scholar]
- Boyd A. W., Wawryk S. O., Burns G. F., Fecondo J. V. Intercellular adhesion molecule 1 (ICAM-1) has a central role in cell-cell contact-mediated immune mechanisms. Proc Natl Acad Sci U S A. 1988 May;85(9):3095–3099. doi: 10.1073/pnas.85.9.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan D. C., Yui M. A., Wuthrich R. P., Kelley V. E. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989 Dec 1;143(11):3470–3475. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dougherty G. J., Hogg N. The role of monocyte lymphocyte function-associated antigen 1 (LFA-1) in accessory cell function. Eur J Immunol. 1987 Jul;17(7):943–947. doi: 10.1002/eji.1830170708. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Dustin M. L., Singer K. H., Tuck D. T., Springer T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon gamma and is mediated by intercellular adhesion molecule 1 (ICAM-1). J Exp Med. 1988 Apr 1;167(4):1323–1340. doi: 10.1084/jem.167.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988 Jul;107(1):321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Griffiths C. E., Voorhees J. J., Nickoloff B. J. Gamma interferon induces different keratinocyte cellular patterns of expression of HLA-DR and DQ and intercellular adhesion molecule-I (ICAM-I) antigens. Br J Dermatol. 1989 Jan;120(1):1–8. doi: 10.1111/j.1365-2133.1989.tb07759.x. [DOI] [PubMed] [Google Scholar]
- Horley K. J., Carpenito C., Baker B., Takei F. Molecular cloning of murine intercellular adhesion molecule (ICAM-1). EMBO J. 1989 Oct;8(10):2889–2896. doi: 10.1002/j.1460-2075.1989.tb08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Dustin M. L., Springer T. A., Clark E. A., Mannoni P., Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B, T and myeloid cells. Nature. 1988 Jan 7;331(6151):86–88. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- Makgoba M. W., Sanders M. E., Ginther Luce G. E., Gugel E. A., Dustin M. L., Springer T. A., Shaw S. Functional evidence that intercellular adhesion molecule-1 (ICAM-1) is a ligand for LFA-1-dependent adhesion in T cell-mediated cytotoxicity. Eur J Immunol. 1988 Apr;18(4):637–640. doi: 10.1002/eji.1830180423. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mentzer S. J., Rothlein R., Springer T. A., Faller D. V. Intercellular adhesion molecule-1 (ICAM-1) is involved in the cytolytic T lymphocyte interaction with a human synovial cell line. J Cell Physiol. 1988 Oct;137(1):173–178. doi: 10.1002/jcp.1041370121. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Rothlein R., Dustin M. L., Marlin S. D., Springer T. A. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986 Aug 15;137(4):1270–1274. [PubMed] [Google Scholar]
- Simmons D., Makgoba M. W., Seed B. ICAM, an adhesion ligand of LFA-1, is homologous to the neural cell adhesion molecule NCAM. Nature. 1988 Feb 18;331(6157):624–627. doi: 10.1038/331624a0. [DOI] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Marlin S. D., Stratowa C., Dustin M. L., Springer T. A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell. 1988 Mar 25;52(6):925–933. doi: 10.1016/0092-8674(88)90434-5. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]
- Takei F. Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J Immunol. 1985 Mar;134(3):1403–1407. [PubMed] [Google Scholar]
- Temponi M., Romano G., D'Urso C. M., Wang Z., Kekish U., Ferrone S. Profile of intercellular adhesion molecule-1 (ICAM-1) synthesized by human melanoma cell lines. Semin Oncol. 1988 Dec;15(6):595–607. [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Wawryk S. O., Novotny J. R., Wicks I. P., Wilkinson D., Maher D., Salvaris E., Welch K., Fecondo J., Boyd A. W. The role of the LFA-1/ICAM-1 interaction in human leukocyte homing and adhesion. Immunol Rev. 1989 Apr;108:135–161. doi: 10.1111/j.1600-065x.1989.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Wuthrich R. P., Yui M. A., Mazoujian G., Nabavi N., Glimcher L. H., Kelley V. E. Enhanced MHC class II expression in renal proximal tubules precedes loss of renal function in MRL/lpr mice with lupus nephritis. Am J Pathol. 1989 Jan;134(1):45–51. [PMC free article] [PubMed] [Google Scholar]