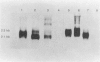
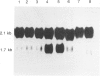
Abstract
Rabbit aortic smooth muscle cells (SMC) and Rb-1 cells, a continuous line of the same origin, were transformed by transfection with pSV3-neo DNA, a plasmid containing the SV40 early region linked to the neoR resistance gene. Transformed clones were selected in G418-containing medium at a rate of 10(-4) per cell. All transformed clones were immortalized and contained in the early passages two free recombined plasmids derived from pSV3-neo. At advanced passages pSV3-neo sequences were found integrated in the cellular genome. Transformed cells had an altered morphology and growth pattern that differed among clones. Some clones reached high density in low-serum medium. All the clones stained positively for the intranuclear T antigen. Some clones had distinct transcripts for the large T and small t antigens, while in others only larger or truncated transcripts were found. Alpha-actin filaments were visualized by immunofluorescent staining in all the clones, but Northern blot analysis revealed a significant reduction in transcripts for this actin. All the transformed clones accumulated, to a variable extent, cholesteryl esters after incubation with beta very low-density lipoprotein. Six of the eight transformed clones maintained a diploid chromosome number, but there was an increase in structural chromosome aberrations, predominantly dicentrics. Transfection of pSV3-neo into rabbit vascular SMCs is an efficient model for obtaining transformed clonal populations. These clones show some phenotypic changes that may be relevant to the study of atherogenesis.
Full text
PDF


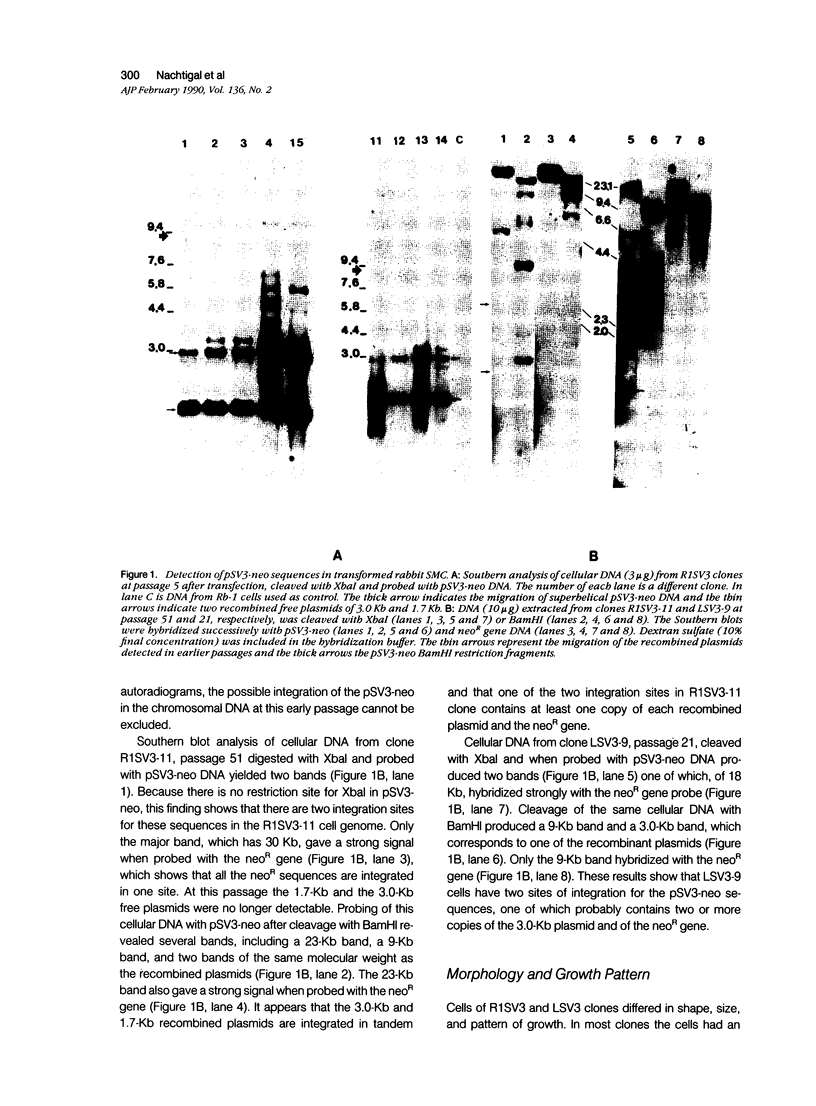




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Vanderlaan M., Burns F. J., Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977 Jul;37(7 Pt 1):2232–2235. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barja F., Coughlin C., Belin D., Gabbiani G. Actin isoform synthesis and mRNA levels in quiescent and proliferating rat aortic smooth muscle cells in vivo and in vitro. Lab Invest. 1986 Aug;55(2):226–233. [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. K., Li L., McClure D. B. Altered low density lipoprotein receptor regulation is associated with cholesteryl ester accumulation in Simian virus 40 transformed rodent fibroblast cell lines. In Vitro Cell Dev Biol. 1988 Apr;24(4):353–358. doi: 10.1007/BF02628838. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cunningham M. J., Pasternak R. C. The potential role of viruses in the pathogenesis of atherosclerosis. Circulation. 1988 May;77(5):964–966. doi: 10.1161/01.cir.77.5.964. [DOI] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant C. G., Hajjar D. P., Minick C. R., Fabricant J. Herpesvirus infection enhances cholesterol and cholesteryl ester accumulation in cultured arterial smooth muscle cells. Am J Pathol. 1981 Nov;105(2):176–184. [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fowler S. D., Brown W. J., Warfel J., Greenspan P. Use of nile red for the rapid in situ quantitation of lipids on thin-layer chromatograms. J Lipid Res. 1987 Oct;28(10):1225–1232. [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeny E. K., Sprague E. A., Schwartz C. J., Gauntt C. J. Coxsackievirus group B replication in cultured fetal baboon aortic smooth muscle cells. J Med Virol. 1986 Oct;20(2):135–149. doi: 10.1002/jmv.1890200206. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Regulation of SV40 gene expression. Adv Cancer Res. 1981;35:111–149. doi: 10.1016/s0065-230x(08)60910-0. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Tjian R., Topp W. C. Simian virus 40 small-t protein is required for loss of actin cable networks in rat cells. J Virol. 1980 Mar;33(3):1182–1191. doi: 10.1128/jvi.33.3.1182-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenspan P., Mayer E. P., Fowler S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985 Mar;100(3):965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Fabricant C. G., Minick C. R., Fabricant J. Virus-induced atherosclerosis. Herpesvirus infection alters aortic cholesterol metabolism and accumulation. Am J Pathol. 1986 Jan;122(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P., Falcone D. J., Fabricant C. G., Fabricant J. Altered cholesteryl ester cycle is associated with lipid accumulation in herpesvirus-infected arterial smooth muscle cells. J Biol Chem. 1985 May 25;260(10):6124–6128. [PubMed] [Google Scholar]
- Hajjar D. P., Pomerantz K. B., Falcone D. J., Weksler B. B., Grant A. J. Herpes simplex virus infection in human arterial cells. Implications in arteriosclerosis. J Clin Invest. 1987 Nov;80(5):1317–1321. doi: 10.1172/JCI113208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Topp W. C., Ozanne B. Simian virus 40 induces the production of a polypeptide transforming factor(s). Virology. 1981 Jan 30;108(2):484–490. doi: 10.1016/0042-6822(81)90455-4. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Leavitt J., Gunning P., Kedes L., Jariwalla R. Smooth muscle alpha-action is a transformation-sensitive marker for mouse NIH 3T3 and Rat-2 cells. 1985 Aug 29-Sep 4Nature. 316(6031):840–842. doi: 10.1038/316840a0. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Getz M. J., Moses H. L. Transforming growth factor type beta regulation of actin mRNA. J Cell Physiol. 1986 Apr;127(1):83–88. doi: 10.1002/jcp.1041270111. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Salomon R. N., Birinyi L. K. Production of platelet-derived growth factor-like mitogen by smooth-muscle cells from human atheroma. N Engl J Med. 1988 Jun 9;318(23):1493–1498. doi: 10.1056/NEJM198806093182303. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Benditt E. P., Juchau M. R. Focal smooth muscle proliferation in the aortic intima produced by an initiation-promotion sequence. Proc Natl Acad Sci U S A. 1985 May;82(10):3450–3454. doi: 10.1073/pnas.82.10.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark-Malchoff D., Marinetti G. V., Hare J. D., Meisler A. Cholesterol content and metabolism in normal and polyoma virus-transformed hamster embryo fibroblasts. Exp Cell Res. 1979 Feb;118(2):377–381. doi: 10.1016/0014-4827(79)90161-7. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Nachtigal M., Greenspan P., Terracio L., Fowler S. D. Transformation of rabbit arterial smooth muscle cells with simian virus 40. Arch Virol. 1987;95(3-4):225–235. doi: 10.1007/BF01310782. [DOI] [PubMed] [Google Scholar]
- Nachtigal M., Nachtigal S., Lungeanu A., Macnab J. C. Chromosome changes in rat embryo cell lines transformed by temperature-sensitive mutants and sheared DNA of herpes simplex virus. Cancer Genet Cytogenet. 1982 Dec;7(4):313–326. doi: 10.1016/0165-4608(82)90048-6. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A., Garte S. J., Warren L., Nesta D., Mindich B. Transforming gene in human atherosclerotic plaque DNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7951–7955. doi: 10.1073/pnas.83.20.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie B. L., Adam E., Melnick J. L. Association of herpesvirus/cytomegalovirus infections with human atherosclerosis. Prog Med Virol. 1988;35:21–42. [PubMed] [Google Scholar]
- Phillips B., Rundell K. Failure of simian virus 40 small t antigen to disorganize actin cables in nonpermissive cell lines. J Virol. 1988 Mar;62(3):768–775. doi: 10.1128/jvi.62.3.768-775.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirozzi S., Young D. V. Evidence for a transforming growth factor(s) in the serum-free conditioned medium of SV40-transformed 3T3 fibroblasts. Int J Cancer. 1984 Apr 15;33(4):525–532. doi: 10.1002/ijc.2910330417. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Shapland C., Lowings P., Lawson D. Identification of new actin-associated polypeptides that are modified by viral transformation and changes in cell shape. J Cell Biol. 1988 Jul;107(1):153–161. doi: 10.1083/jcb.107.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Smith E. B. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12(0):1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Strauch A. R., Rubenstein P. A. Induction of vascular smooth muscle alpha-isoactin expression in BC3H1 cells. J Biol Chem. 1984 Mar 10;259(5):3152–3159. [PubMed] [Google Scholar]
- Thomas W. A., Kim D. N. Biology of disease. Atherosclerosis as a hyperplastic and/or neoplastic process. Lab Invest. 1983 Mar;48(3):245–255. [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt D. P., Brown D. J., Gordon J. A. Transformation-sensitive isoactin in passaged chick embryo fibroblasts transformed by Rous sarcoma virus. J Cell Biol. 1983 Jun;96(6):1766–1771. doi: 10.1083/jcb.96.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiroya H. M., Ghosh L., Yang R., Robertson A. L., Jr Herpesviridae in the coronary arteries and aorta of young trauma victims. Am J Pathol. 1988 Jan;130(1):71–79. [PMC free article] [PubMed] [Google Scholar]