Abstract
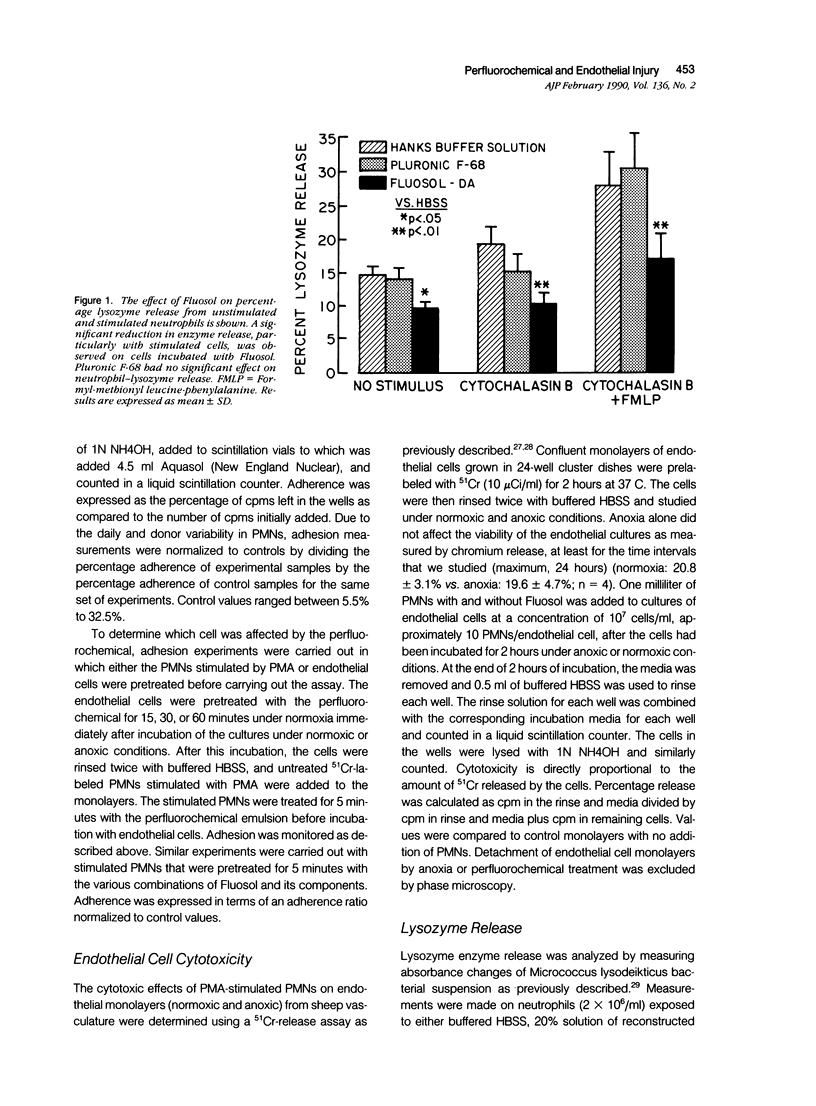
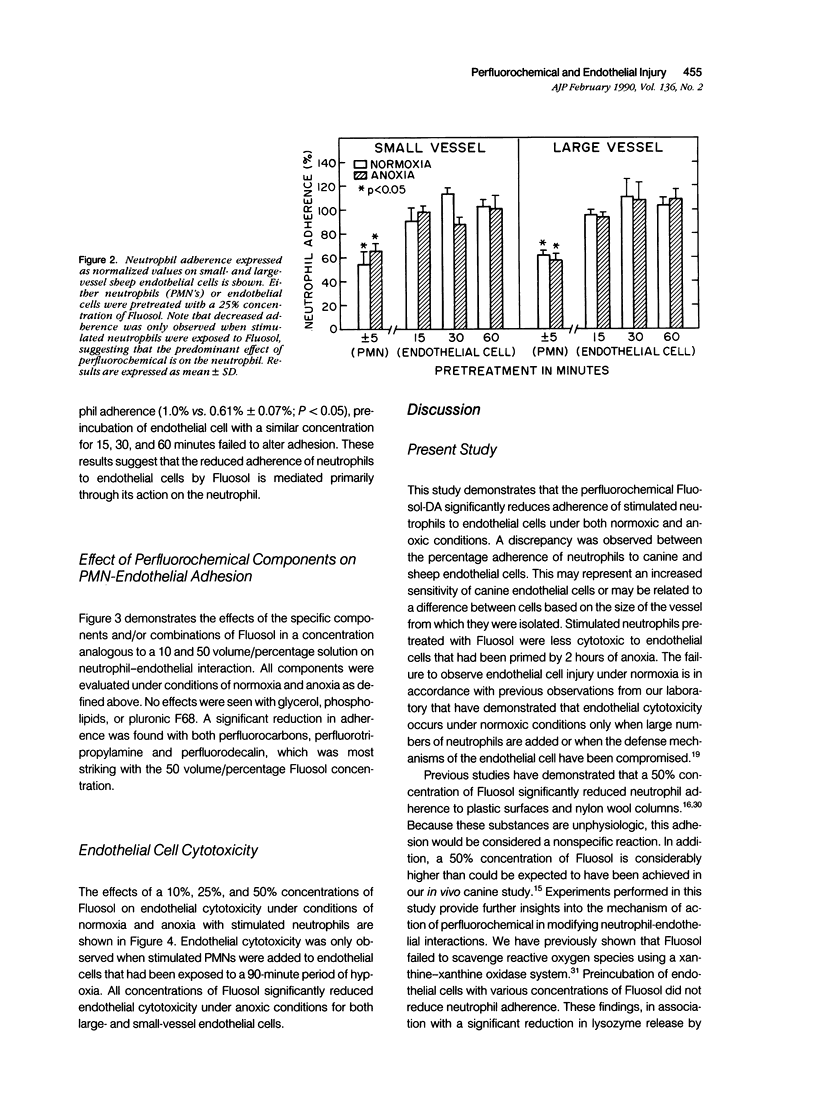
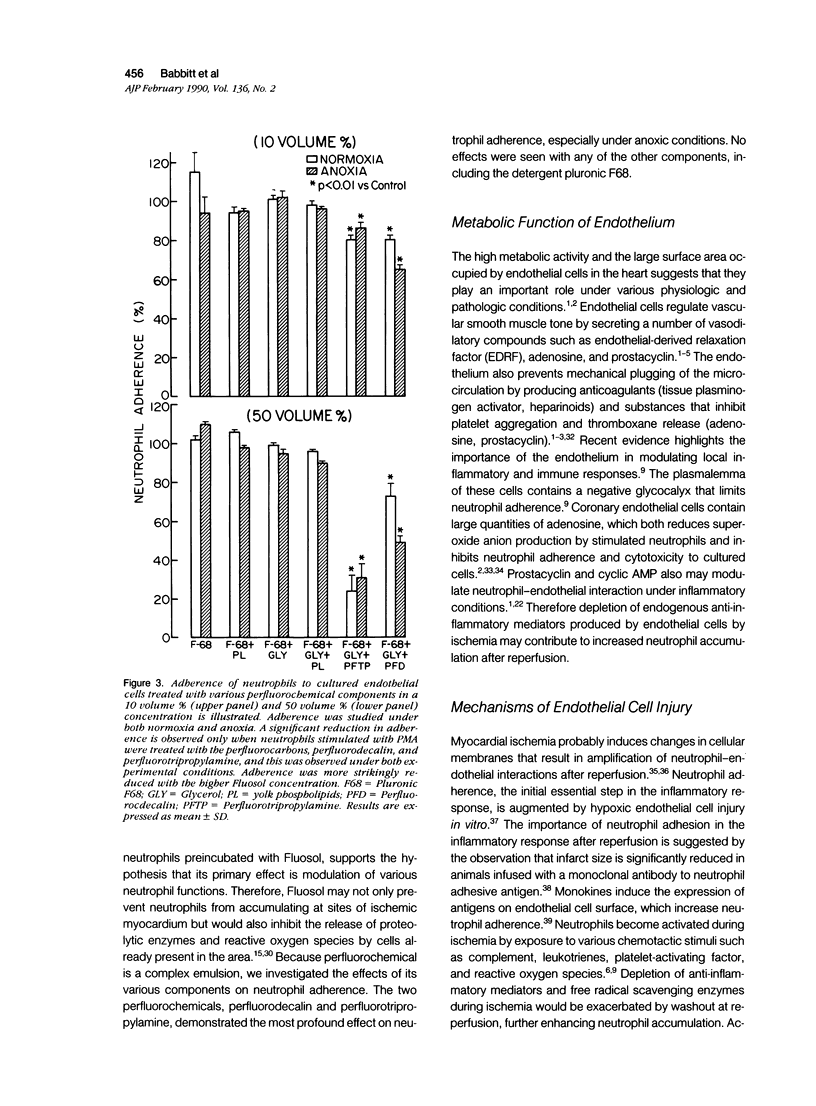
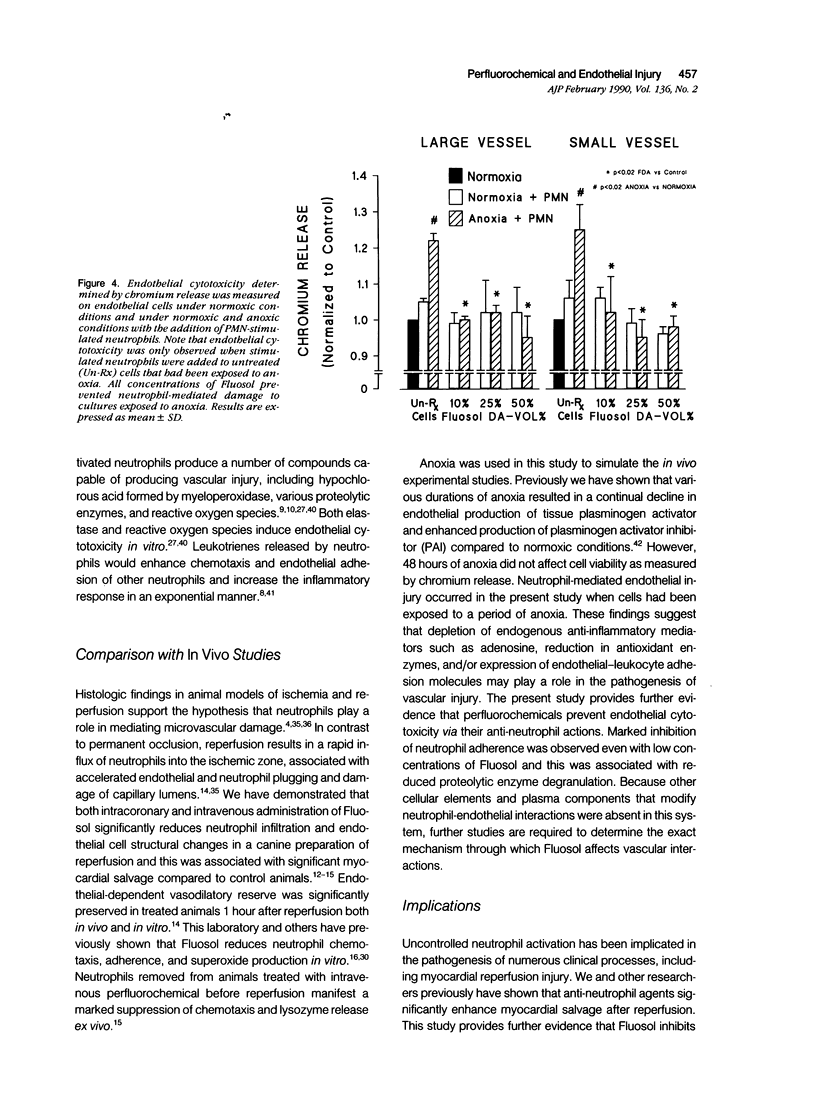
Myocardial salvage after reperfusion may be limited by neutrophil-mediated microvascular damage. The effect of the perfluorochemical, Fluosol-DA, and its various components on neutrophil adherence, cytotoxicity, and proteolytic enzyme release was examined on sheep large and small vessel endothelial cells in vitro. Cells were studied under normoxic (N) and anoxic conditions (A). Various concentrations of Fluosol (10%, 25%, and 50%) significantly reduced neutrophil adherence under both experimental conditions [mean 22 +/- 3.25% versus 7 +/- 0.8% (N) and 20 +/- 3.2% versus 7.5 +/- 0.9% (A); P less than 0.01]. The perfluorocarbons, perfluorodecalin (PFD), and perfluoro-tripropylamine (PFTP) in a 50 volume/percent concentration exhibited profound effects on adherence, particularly on cells subjected to anoxia (51% and 69% reduction in adherence, respectively; P less than 0.01). No effect on adherence was observed with other components, including the detergent, pluronic F68. A 25% reduction (P less than 0.02) in endothelial cytotoxicity was noted when neutrophils were preincubated with Fluosol. However, pretreatment of endothelial cells with Fluosol did not inhibit neutrophil adherence. Neutrophils stimulated with cytochalasin B and FMLP showed a significant reduction in lysozyme release after incubation with Fluosol (28 +/- 5% versus 17 +/- 4%; P less than 0.01). This study demonstrates that Fluosol significantly attenuates neutrophil adherence, cytotoxicity, and enzyme release in an in vitro model of microvascular injury. It also suggests that prevention of neutrophil-mediated microvascular damage may be an important mechanism whereby Fluosol enhances myocardial salvage after ischemia and reperfusion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. W., Gorman R. R., Shebuski R. J. Prevention of blockage of partially obstructed coronary arteries with prostacyclin correlates with inhibition of platelet aggregation. Prostaglandins. 1979 Apr;17(4):483–494. doi: 10.1016/0090-6980(79)90001-7. [DOI] [PubMed] [Google Scholar]
- Bajaj A. K., Cobb M. A., Virmani R., Gay J. C., Light R. T., Forman M. B. Limitation of myocardial reperfusion injury by intravenous perfluorochemicals. Role of neutrophil activation. Circulation. 1989 Mar;79(3):645–656. doi: 10.1161/01.cir.79.3.645. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Bowman C. M., Butler E. N., Repine J. E. Hyperoxia damages cultured endothelial cells causing increased neutrophil adherence. Am Rev Respir Dis. 1983 Sep;128(3):469–472. doi: 10.1164/arrd.1983.128.3.469. [DOI] [PubMed] [Google Scholar]
- Braquet P., Touqui L., Shen T. Y., Vargaftig B. B. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987 Jun;39(2):97–145. [PubMed] [Google Scholar]
- Cronstein B. N., Kramer S. B., Weissmann G., Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983 Oct 1;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Levin R. I., Belanoff J., Weissmann G., Hirschhorn R. Adenosine: an endogenous inhibitor of neutrophil-mediated injury to endothelial cells. J Clin Invest. 1986 Sep;78(3):760–770. doi: 10.1172/JCI112638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res. 1982 Oct;51(4):439–447. doi: 10.1161/01.res.51.4.439. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Forman M. B., Bingham S., Kopelman H. A., Wehr C., Sandler M. P., Kolodgie F., Vaughn W. K., Friesinger G. C., Virmani R. Reduction of infarct size with intracoronary perfluorochemical in a canine preparation of reperfusion. Circulation. 1985 May;71(5):1060–1068. doi: 10.1161/01.cir.71.5.1060. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Puett D. W., Bingham S. E., Virmani R., Tantengco M. V., Light R. T., Bajaj A., Price R., Friesinger G. Preservation of endothelial cell structure and function by intracoronary perfluorochemical in a canine preparation of reperfusion. Circulation. 1987 Aug;76(2):469–479. doi: 10.1161/01.cir.76.2.469. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Puett D. W., Wilson B. H., Vaughn W. K., Friesinger G. C., Virmani R. Beneficial long-term effect of intracoronary perfluorochemical on infarct size and ventricular function in a canine reperfusion model. J Am Coll Cardiol. 1987 May;9(5):1082–1090. doi: 10.1016/s0735-1097(87)80311-x. [DOI] [PubMed] [Google Scholar]
- Gerlach E., Nees S., Becker B. F. The vascular endothelium: a survey of some newly evolving biochemical and physiological features. Basic Res Cardiol. 1985 Sep-Oct;80(5):459–474. doi: 10.1007/BF01907911. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Hoover R. L., Briggs R. T., Karnovsky M. J. The adhesive interaction between polymorphonuclear leukocytes and endothelial cells in vitro. Cell. 1978 Jun;14(2):423–428. doi: 10.1016/0092-8674(78)90127-7. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Karnovsky M. J., Austen K. F., Corey E. J., Lewis R. A. Leukotriene B4 action on endothelium mediates augmented neutrophil/endothelial adhesion. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2191–2193. doi: 10.1073/pnas.81.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover R. L., Robinson J. M., Karnovsky M. J. Adhesion of polymorphonuclear leukocytes to endothelium enhances the efficiency of detoxification of oxygen-free radicals. Am J Pathol. 1987 Feb;126(2):258–268. [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R. Human pulmonary endothelial cells in culture. Activities of cells from arteries and cells from veins. J Clin Invest. 1980 Apr;65(4):841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser L., Sparks H. V., Jr Endothelial cells. Not just a cellophane wrapper. Arch Intern Med. 1987 Mar;147(3):569–573. doi: 10.1001/archinte.147.3.569. [DOI] [PubMed] [Google Scholar]
- Lane T. A., Lamkin G. E. Paralysis of phagocyte migration due to an artificial blood substitute. Blood. 1984 Aug;64(2):400–405. [PubMed] [Google Scholar]
- Mullane K. M., Salmon J. A., Kraemer R. Leukocyte-derived metabolites of arachidonic acid in ischemia-induced myocardial injury. Fed Proc. 1987 May 15;46(7):2422–2433. [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Rossen R. D., Swain J. L., Michael L. H., Weakley S., Giannini E., Entman M. L. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle. A possible initiator of an extra myocardial mechanism of ischemic injury. Circ Res. 1985 Jul;57(1):119–130. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Schultz D. R., Ruan J. W. Fc and C3b receptors on pulmonary endothelial cells: induction by injury. Science. 1981 Oct 30;214(4520):557–558. doi: 10.1126/science.6270789. [DOI] [PubMed] [Google Scholar]
- SMOLELIS A. N., HARTSELL S. E. The determination of lysozyme. J Bacteriol. 1949 Dec;58(6):731–736. doi: 10.1128/jb.58.6.731-736.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Fantone J. C., Mickelson J. K., Griffin J. D., Lucchesi B. R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988 Feb;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedly L. A., Tonnesen M. G., Sandhaus R. A., Haslett C., Guthrie L. A., Johnston R. B., Jr, Henson P. M., Worthen G. S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986 Apr;77(4):1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub M., Sato G. Growth of functional primary cultures of kidney epithelial cells in defined medium. J Cell Physiol. 1980 Nov;105(2):369–378. doi: 10.1002/jcp.1041050220. [DOI] [PubMed] [Google Scholar]
- Virmani R., Fink L. M., Gunter K., English D. Effect of perfluorochemical blood substitutes on human neutrophil function. Transfusion. 1984 Jul-Aug;24(4):343–347. doi: 10.1046/j.1537-2995.1984.24484275579.x. [DOI] [PubMed] [Google Scholar]
- Wagner R. C., Matthews M. A. The isolation and culture of capillary endothelium from epididymal fat. Microvasc Res. 1975 Nov;10(3):286–297. doi: 10.1016/0026-2862(75)90033-3. [DOI] [PubMed] [Google Scholar]
- Walther B. T., Ohman R., Roseman S. A quantitative assay for intercellular adhesion. Proc Natl Acad Sci U S A. 1973 May;70(5):1569–1573. doi: 10.1073/pnas.70.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojta J., Jones R. L., Binder B. R., Hoover R. L. Reduction in pO2 decreases the fibrinolytic potential of cultured bovine endothelial cells derived from pulmonary arteries and lung microvasculature. Blood. 1988 Jun;71(6):1703–1706. [PubMed] [Google Scholar]



