Abstract
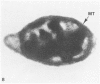
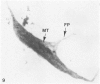
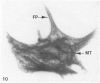
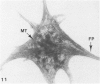
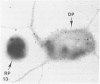
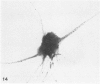
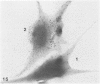
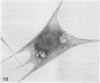
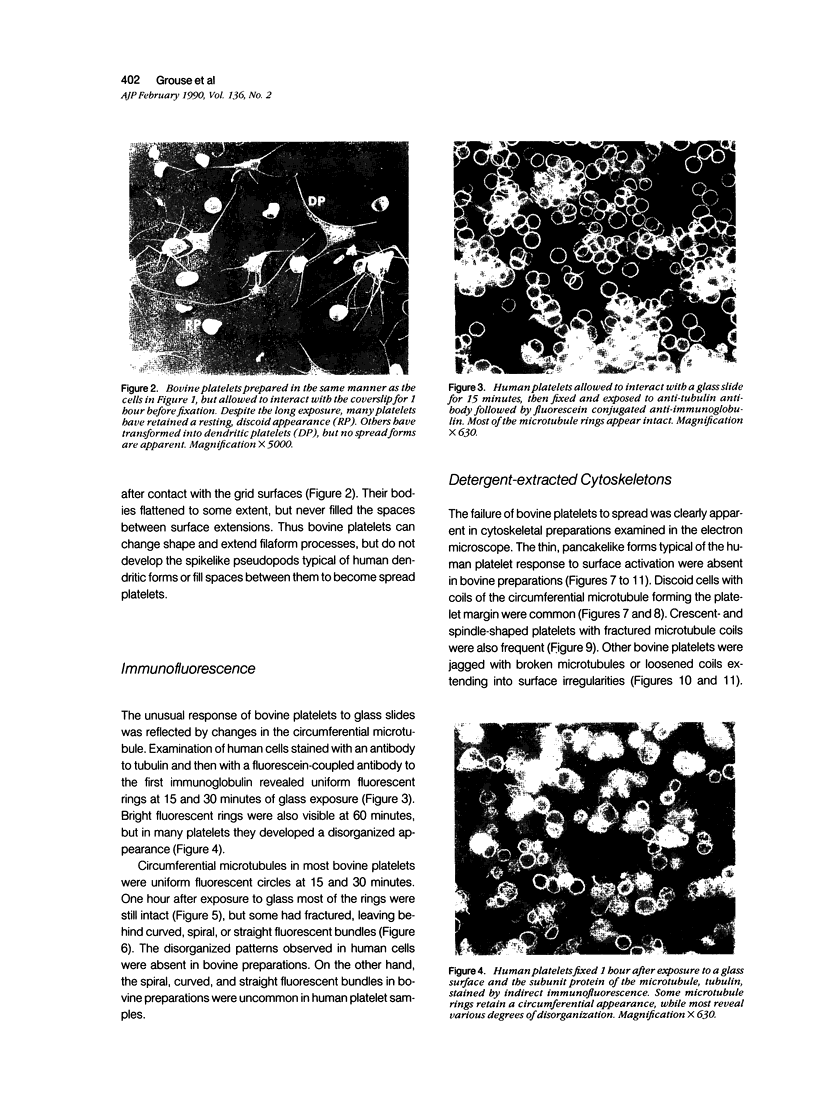
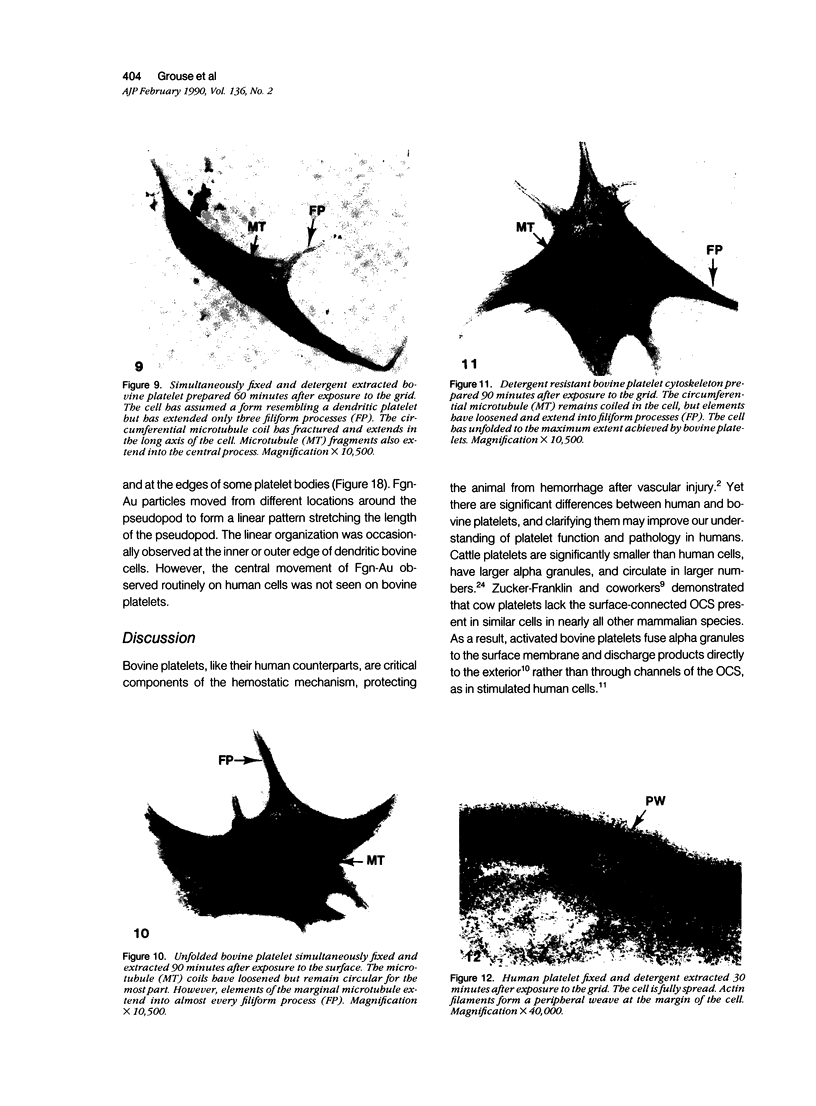
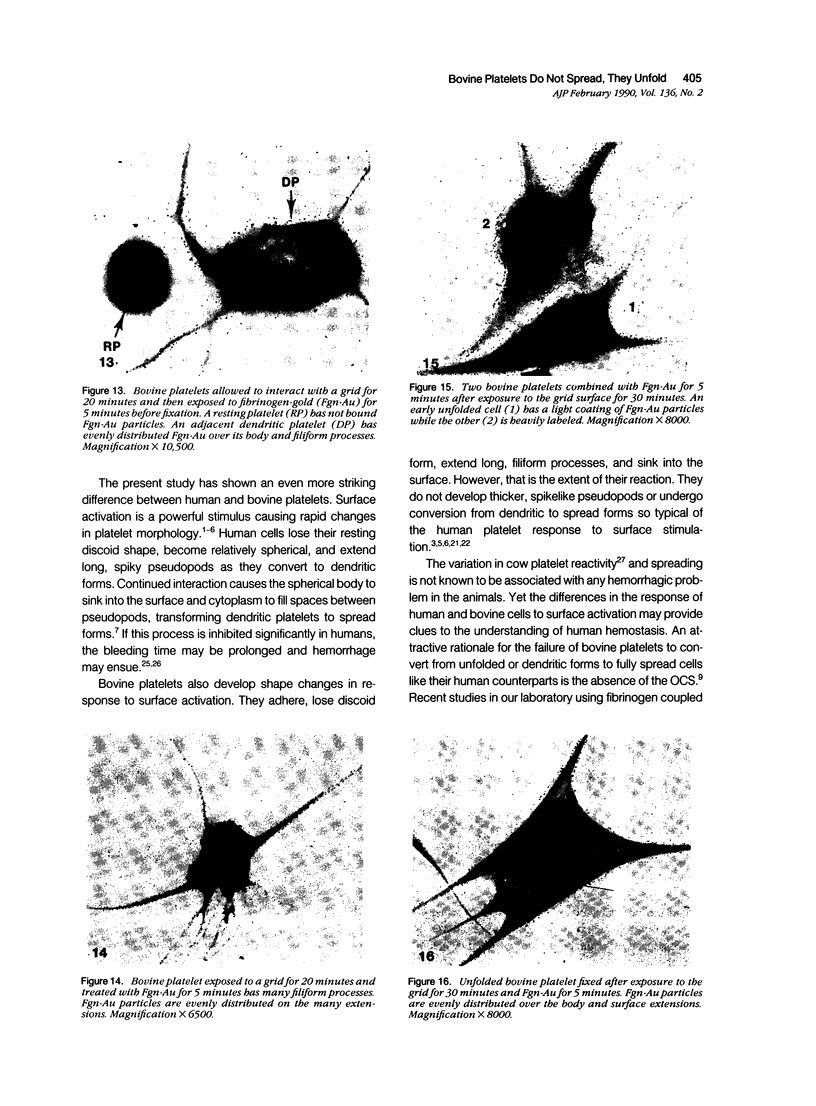
The present study has examined the response of bovine platelets to surface activation and compared it to the reaction of human cells. Human platelets react to surfaces by losing their discoid shape, extending pseudopods, converting to dendritic forms, and finally, spreading into thin films resembling pancakes. Bovine platelets do not spread, they unfold. Surface activation causes them to transform from discs to irregular, flattened shapes resembling dendritic platelets, but they are unable to fill in spaces between pseudopods, a step required for spreading. Bovine platelets lack the surface-connected open canalicular system (OCS), which serves as a reservoir of membrane for human platelet spreading. Its absence may be the major factor in the failure of bovine platelet spreading, but there are other possible factors. Circumferential microtubules are more resistant to disassembly in surface-activated bovine than human cells, and their stability as rings or fractured bundles may limit spreading. Actin filament assembly is similar in human and bovine platelets, but the organization is different. Human platelets form a peripheral weave of actin that expands the membrane between pseudopods. A peripheral weave does not form in surface-activated bovine platelets. The absence of the OCS and differences in cytoskeletal organization in bovine platelets may also affect spreading of the surface membrane. Fibrinogen-gold (Fgn-Au) probes added to spread human platelet move from pseudopods and the cell margin toward the center and concentrate in the OCS. Fgn-Au particles bind to surface-activated bovine cells, but move very little, or not at all. All of these factors may contribute to the inability of bovine platelets to react to surfaces by spreading like human cells, but absence of the OCS appears to be the major cause.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke O., Bray D. Surface movements during the spreading of blood platelets. Eur J Cell Biol. 1988 Jun;46(2):207–216. [PubMed] [Google Scholar]
- Escolar G., Krumwiede M., White J. G. Organization of the actin cytoskeleton of resting and activated platelets in suspension. Am J Pathol. 1986 Apr;123(1):86–94. [PMC free article] [PubMed] [Google Scholar]
- Geoghegan W. D., Ackerman G. A. Adsorption of horseradish peroxidase, ovomucoid and anti-immunoglobulin to colloidal gold for the indirect detection of concanavalin A, wheat germ agglutinin and goat anti-human immunoglobulin G on cell surfaces at the electron microscopic level: a new method, theory and application. J Histochem Cytochem. 1977 Nov;25(11):1187–1200. doi: 10.1177/25.11.21217. [DOI] [PubMed] [Google Scholar]
- Goodman S. L., Albrecht R. M. Correlative light and electron microscopy of platelet adhesion and fibrinogen receptor expression using colloidal-gold labeling. Scanning Microsc. 1987 Jun;1(2):727–734. [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Höglund A. S., Karlsson R., Arro E., Fredriksson B. A., Lindberg U. Visualization of the peripheral weave of microfilaments in glia cells. J Muscle Res Cell Motil. 1980 Jun;1(2):127–146. doi: 10.1007/BF00711795. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Lassing I., Höglund A. S., Lindberg U. The organization of microfilaments in spreading platelets: a comparison with fibroblasts and glial cells. J Cell Physiol. 1984 Oct;121(1):96–113. doi: 10.1002/jcp.1041210113. [DOI] [PubMed] [Google Scholar]
- Loftus J. C., Albrecht R. M. Redistribution of the fibrinogen receptor of human platelets after surface activation. J Cell Biol. 1984 Sep;99(3):822–829. doi: 10.1083/jcb.99.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus J. C., Choate J., Albrecht R. M. Platelet activation and cytoskeletal reorganization: high voltage electron microscopic examination of intact and Triton-extracted whole mounts. J Cell Biol. 1984 Jun;98(6):2019–2025. doi: 10.1083/jcb.98.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. A., Albrecht R. M. Colloidal gold labelling of fibrinogen receptors in epinephrine- and ADP-activated platelet suspensions. Scanning Microsc. 1987 Jun;1(2):745–756. [PubMed] [Google Scholar]
- Small J. V. Organization of actin in the leading edge of cultured cells: influence of osmium tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981 Dec;91(3 Pt 1):695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. M., 2nd, Burris S. M., Weiss D. J., White J. G. Comparison of bovine and human platelet deformability, using micropipette elastimetry. Am J Vet Res. 1989 Jan;50(1):34–38. [PubMed] [Google Scholar]
- White J. G. An overview of platelet structural physiology. Scanning Microsc. 1987 Dec;1(4):1677–1700. [PubMed] [Google Scholar]
- White J. G. Arrangements of actin filaments in the cytoskeleton of human platelets. Am J Pathol. 1984 Nov;117(2):207–217. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Burris S. M., Tukey D., Smith C., 2nd, Clawson C. C. Micropipette aspiration of human platelets: influence of microtubules and actin filaments on deformability. Blood. 1984 Jul;64(1):210–214. [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- White J. G., Gerrard J. M. Ultrastructural features of abnormal blood platelets. A review. Am J Pathol. 1976 Jun;83(3):589–632. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Krumwiede M. Further studies of the secretory pathway in thrombin-stimulated human platelets. Blood. 1987 Apr;69(4):1196–1203. [PubMed] [Google Scholar]
- White J. G., Krumwiede M. Influence of cytochalasin B on the shape change induced in platelets by cold. Blood. 1973 Jun;41(6):823–832. [PubMed] [Google Scholar]
- White J. G., Rao G. H. Influence of a microtubule stabilizing agent on platelet structural physiology. Am J Pathol. 1983 Aug;112(2):207–217. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Sauk J. J. Microtubule coils in spread blood platelets. Blood. 1984 Aug;64(2):470–478. [PubMed] [Google Scholar]
- White J. G. The secretory pathway of bovine platelets. Blood. 1987 Mar;69(3):878–885. [PubMed] [Google Scholar]
- Zucker-Franklin D., Benson K. A., Myers K. M. Absence of a surface-connected canalicular system in bovine platelets. Blood. 1985 Jan;65(1):241–244. [PubMed] [Google Scholar]
- Zucker M. B., Nachmias V. T. Platelet activation. Arteriosclerosis. 1985 Jan-Feb;5(1):2–18. doi: 10.1161/01.atv.5.1.2. [DOI] [PubMed] [Google Scholar]