Abstract
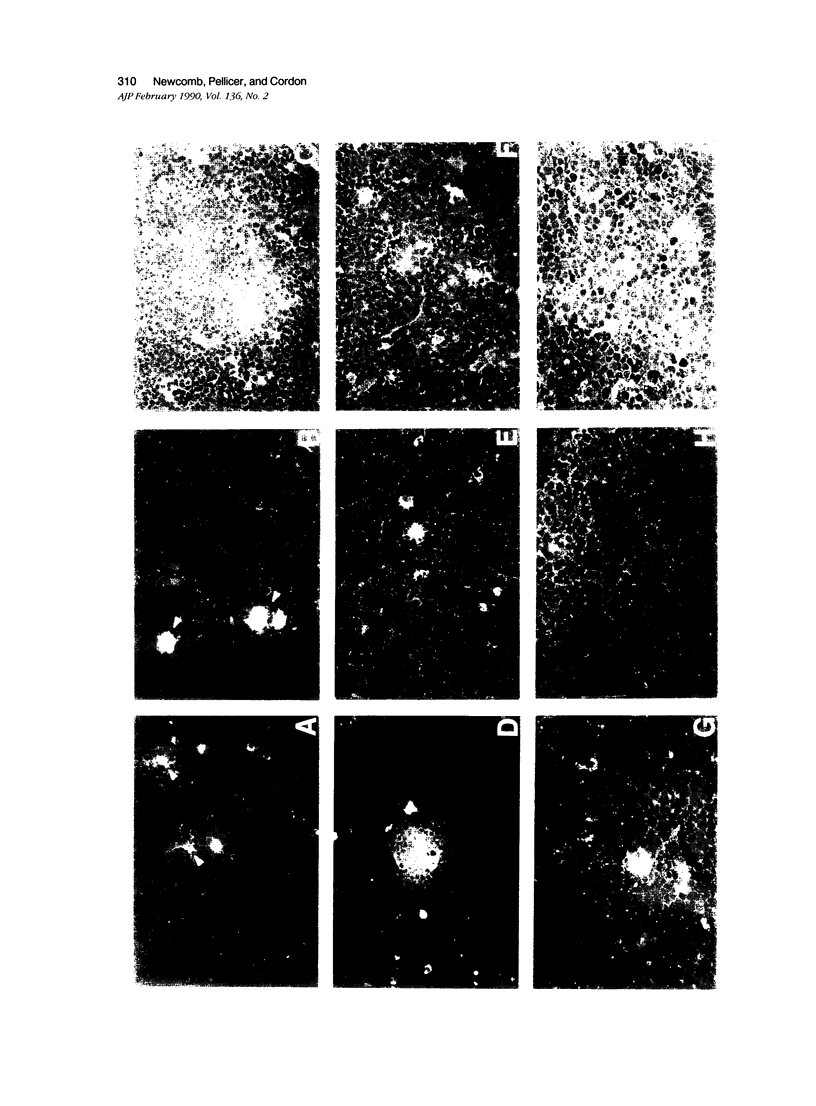
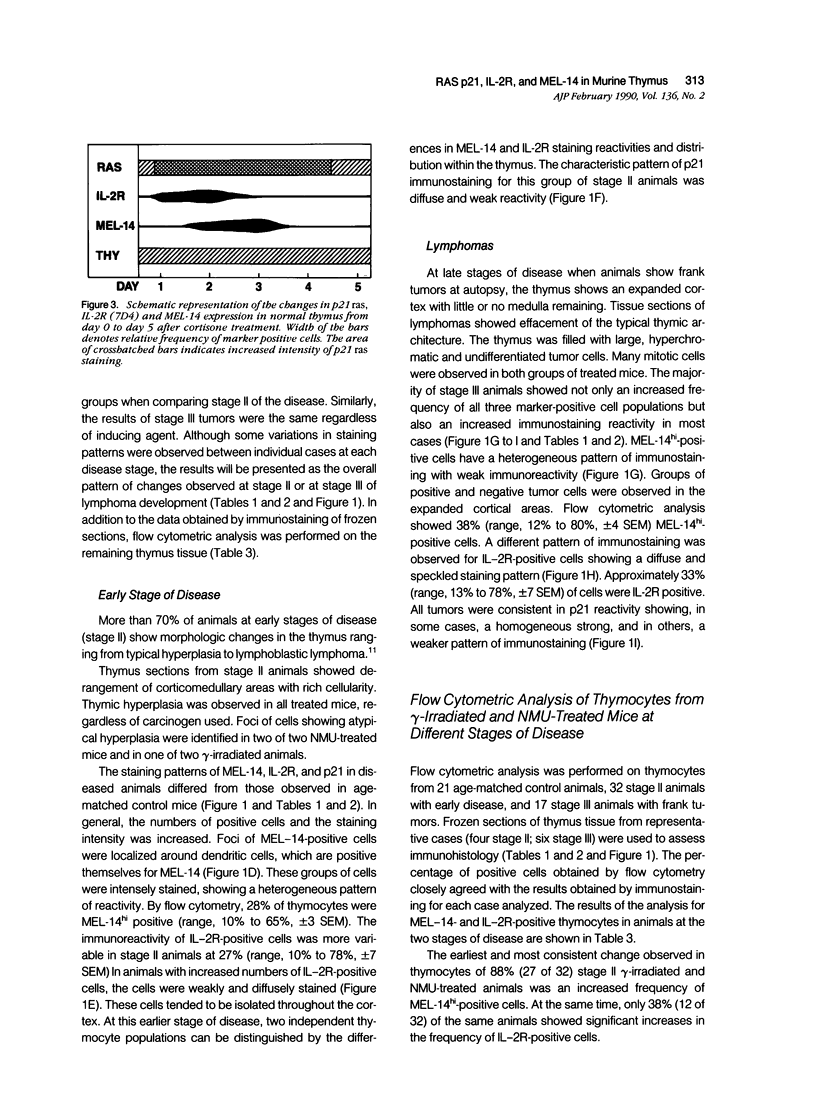
The distribution and localization of thymocytes positive for p21 ras, the lymphocyte homing receptor antigen MEL-14, and IL-2 receptors were studied by immunohistology and flow cytometry. Comparisons were made between age-matched normal mice, carcinogen-treated mice at early (stage II) and late (stage III) stages of disease, and cortisone-treated mice. In normal thymus, the majority of cortical and medullary thymocytes are p21 ras positive. MEL-14hi- and IL-2R-positive cells are located in the cortex and comprise less than 5% of the thymus population. Stage II carcinogen-treated animals consistently show increased numbers of MEL-14hi cells in the thymus, with fewer animals having increased numbers of IL-2R positive cells. These populations appear to be different from one another. All stage III animals have MEL-14hi-positive tumor cells, which in 70% of the cases also express IL-2R. Cortisone treatment was used to study non-malignant proliferation. After cortisone treatment there is a marked increase of p21 ras staining in both the cortex and medulla during the first 72-hour interval. Within 24 hours, 50% of the thymocytes are IL-2R positive, but MEL-14hi cells are not detected. By 48 hours, 90% of the thymus population expresses IL-2R and 50% of the cells are MEL-14hi positive, and this results in a substantial population of cells positive for both IL-2R positive:MEL-14hi markers. This population rapidly disappears by 72 hours, leaving 90% of the cells MEL-14hi positive and less than 10% IL-2R positive. The staining of p21 ras at 72 hours is unusual, showing a speckled, cytoplasmic pattern. In light of our findings, we propose that the first step in thymic lymphomagenesis in carcinogen-treated C57BL/6 mice involves the rare cortical MEL-14hi subpopulation and is thymic dependent. A late stage involves expression of IL-2 receptors by a subset of MEL-14hi cells, thus conferring the potential for autonomous growth and malignancy.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amari N. M., Meruelo D. Murine thymomas induced by fractionated-X-irradiation have specific T-cell receptor rearrangements and characteristics associated with day-15 to -16 fetal thymocytes. Mol Cell Biol. 1987 Dec;7(12):4159–4168. doi: 10.1128/mcb.7.12.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boniver J., Simar L. J., Couroy R., Betz E. H. Quantitative analysis of thymus lymphoid cells during murine radioleukemogenesis. Cancer Res. 1978 Jan;38(1):52–58. [PubMed] [Google Scholar]
- Brelińska R., Seidel H. J., Warchoł J. B., Kreja L. Murine thymic multicellular complexes during latency period after methylnitrosourea (MNU) injection. Thymus. 1988;11(2):67–75. [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Finstad C. L., Bander N. H., Melamed M. R. Immunoanatomic distribution of cytostructural and tissue-associated antigens in the human urinary tract. Am J Pathol. 1987 Feb;126(2):269–284. [PMC free article] [PubMed] [Google Scholar]
- Diamantstein T., Osawa H., Graf L., Schirrmacher V. Studies on interleukin 2 receptor expression and IL-2 production by murine T cell lymphomas. Br J Cancer. 1985 Jan;51(1):23–30. doi: 10.1038/bjc.1985.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Gallatin W. M., Reichert R. A., Butcher E. C., Weissman I. L. Homing receptor-bearing thymocytes, an immunocompetent cortical subpopulation. Nature. 1985 Jan 17;313(5999):233–235. doi: 10.1038/313233a0. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Maitra S. C. Bone marrow and thymus regeneration is a condition for thymoma development. Chem Biol Interact. 1974 Jul;9(1):65–69. doi: 10.1016/0009-2797(74)90068-4. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Aldrich T. H., Cordon-Cardo C. Expression of ras proto-oncogene proteins in normal human tissues. Oncogene. 1987 Mar;1(1):47–58. [PubMed] [Google Scholar]
- Furth M. E., Davis L. J., Fleurdelys B., Scolnick E. M. Monoclonal antibodies to the p21 products of the transforming gene of Harvey murine sarcoma virus and of the cellular ras gene family. J Virol. 1982 Jul;43(1):294–304. doi: 10.1128/jvi.43.1.294-304.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallatin M., St John T. P., Siegelman M., Reichert R., Butcher E. C., Weissman I. L. Lymphocyte homing receptors. Cell. 1986 Mar 14;44(5):673–680. doi: 10.1016/0092-8674(86)90832-9. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Gallick G. E., Kurzrock R., Kloetzer W. S., Arlinghaus R. B., Gutterman J. U. Expression of p21ras in fresh primary and metastatic human colorectal tumors. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1795–1799. doi: 10.1073/pnas.82.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Scheid M., Watson J. Biochemical and biologic characterization of lymphocyte regulatory molecules. III. The isolation and phenotypic characterization of Interleukin-2 producing T cell lymphomas. J Immunol. 1980 Dec;125(6):2570–2578. [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Pellicer A. Mutational activation of oncogenes in animal model systems of carcinogenesis. Mutat Res. 1987 May;185(3):293–308. doi: 10.1016/0165-1110(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Houben-Defresne M. P., Varlet A., Boniver J. Thymic nurse cells and thymic repopulation after whole body sublethal irradiation in mice. Thymus. 1984;6(5):324–333. [PubMed] [Google Scholar]
- Joshi V. V., Frei J. V. Gross and microscopic changes in the lymphoreticular system during genesis of malignant lymphoma induced by a single injection of methylnitrosourea in adult mice. J Natl Cancer Inst. 1970 Feb;44(2):379–394. [PubMed] [Google Scholar]
- Kyewski B. A., Rouse R. V., Kaplan H. S. Thymocyte rosettes: multicellular complexes of lymphocytes and bone marrow-derived stromal cells in the mouse thymus. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5646–5650. doi: 10.1073/pnas.79.18.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL J. D., BUFFETT R. F., FURTH J. Pathogenesis of mouse viral leukemia. Proc Soc Exp Biol Med. 1961 Mar;106:455–459. doi: 10.3181/00379727-106-26368. [DOI] [PubMed] [Google Scholar]
- Leon J., Guerrero I., Pellicer A. Differential expression of the ras gene family in mice. Mol Cell Biol. 1987 Apr;7(4):1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorrow L. E., Newcomb E. W., Pellicer A. Identification of a specific marker chromosome early in tumor development in gamma-irradiated C57BL/6J mice. Leukemia. 1988 Feb;2(2):115–119. [PubMed] [Google Scholar]
- Muto M., Kubo E., Sado T. Cellular events during radiation-induced thymic leukemogenesis in mice: abnormal T cell differentiation in the thymus and defect of thymocyte precursors in the bone marrow after split-dose irradiation. J Immunol. 1985 Mar;134(3):2026–2031. [PubMed] [Google Scholar]
- Newcomb E. W., Steinberg J. J., Pellicer A. ras oncogenes and phenotypic staging in N-methylnitrosourea- and gamma-irradiation-induced thymic lymphomas in C57BL/6J mice. Cancer Res. 1988 Oct 1;48(19):5514–5521. [PubMed] [Google Scholar]
- Reichert R. A., Gallatin W. M., Butcher E. C., Weissman I. L. A homing receptor-bearing cortical thymocyte subset: implications for thymus cell migration and the nature of cortisone-resistant thymocytes. Cell. 1984 Aug;38(1):89–99. doi: 10.1016/0092-8674(84)90529-4. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scollay R., Shortman K. Thymocyte subpopulations: an experimental review, including flow cytometric cross-correlations between the major murine thymocyte markers. Thymus. 1983 Sep;5(5-6):245–295. [PubMed] [Google Scholar]
- Scott M. L., Feinberg M. B., Fry K. E., Percy D. E., Lieberman M. Patterns of thymocyte differentiation markers on virus and radiation induced lymphomas of C57BL/Ka mice. Int J Radiat Oncol Biol Phys. 1985 Jan;11(1):71–78. doi: 10.1016/0360-3016(85)90364-5. [DOI] [PubMed] [Google Scholar]
- Seidel H. J., Kreja L. Leukemia induction by methylnitrosourea (MNU) in selected mouse strains. Effects of MNU on hemopoietic stem cells, the immune system and natural killer cells. J Cancer Res Clin Oncol. 1984;108(2):214–220. doi: 10.1007/BF00402469. [DOI] [PubMed] [Google Scholar]
- Takacs L., Osawa H., Diamantstein T. Detection and localization by the monoclonal anti-interleukin 2 receptor antibody AMT-13 of IL 2 receptor-bearing cells in the developing thymus of the mouse embryo and in the thymus of cortisone-treated mice. Eur J Immunol. 1984 Dec;14(12):1152–1156. doi: 10.1002/eji.1830141217. [DOI] [PubMed] [Google Scholar]
- Van Ewijk W. Immunohistology of lymphoid and non-lymphoid cells in the thymus in relation to T lymphocyte differentiation. Am J Anat. 1984 Jul;170(3):311–330. doi: 10.1002/aja.1001700307. [DOI] [PubMed] [Google Scholar]
- Viola M. V., Fromowitz F., Oravez S., Deb S., Schlom J. ras Oncogene p21 expression is increased in premalignant lesions and high grade bladder carcinoma. J Exp Med. 1985 May 1;161(5):1213–1218. doi: 10.1084/jem.161.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Thymic nurse cells--Ia-bearing epithelium involved in T-lymphocyte differentiation? Nature. 1980 Jan 24;283(5745):402–404. doi: 10.1038/283402a0. [DOI] [PubMed] [Google Scholar]