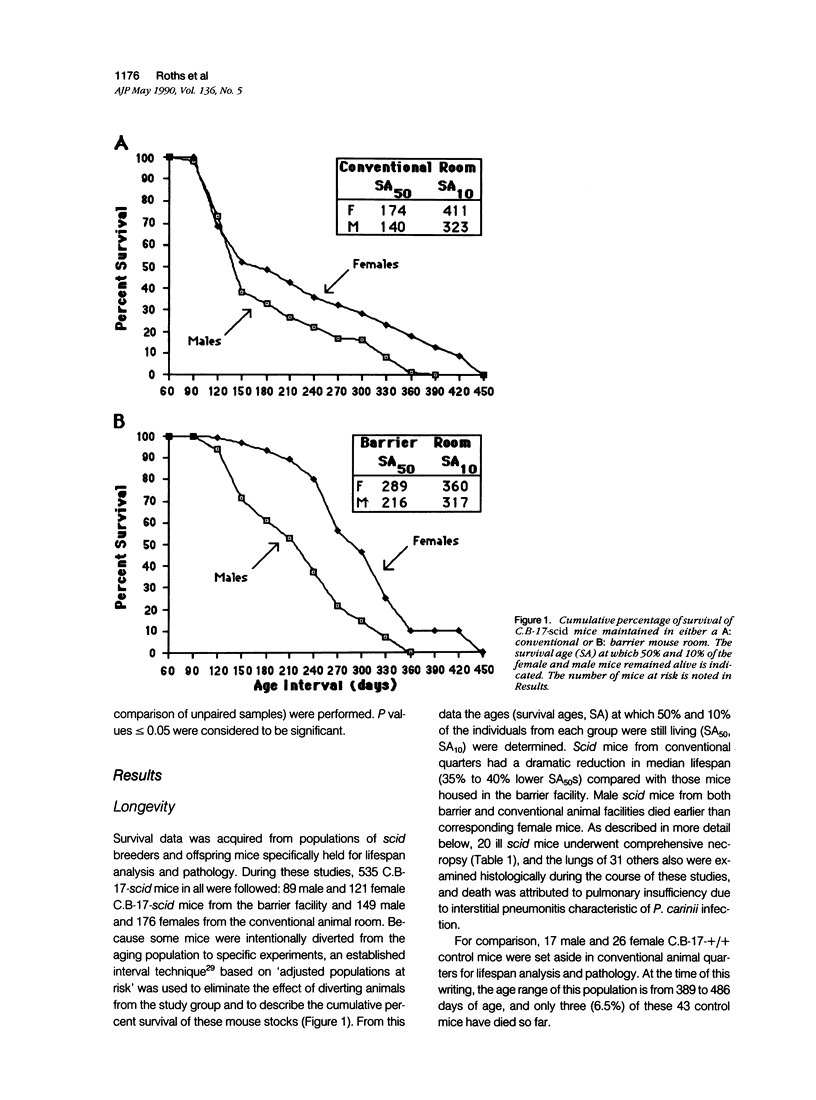
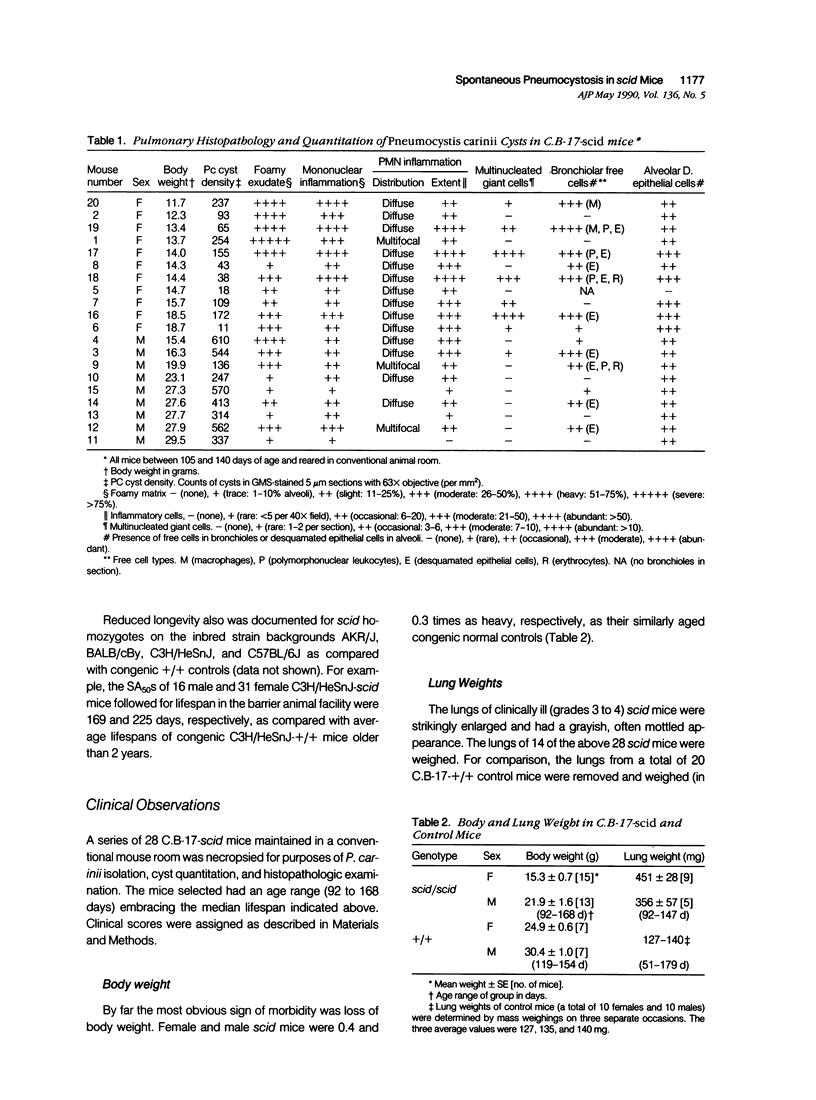
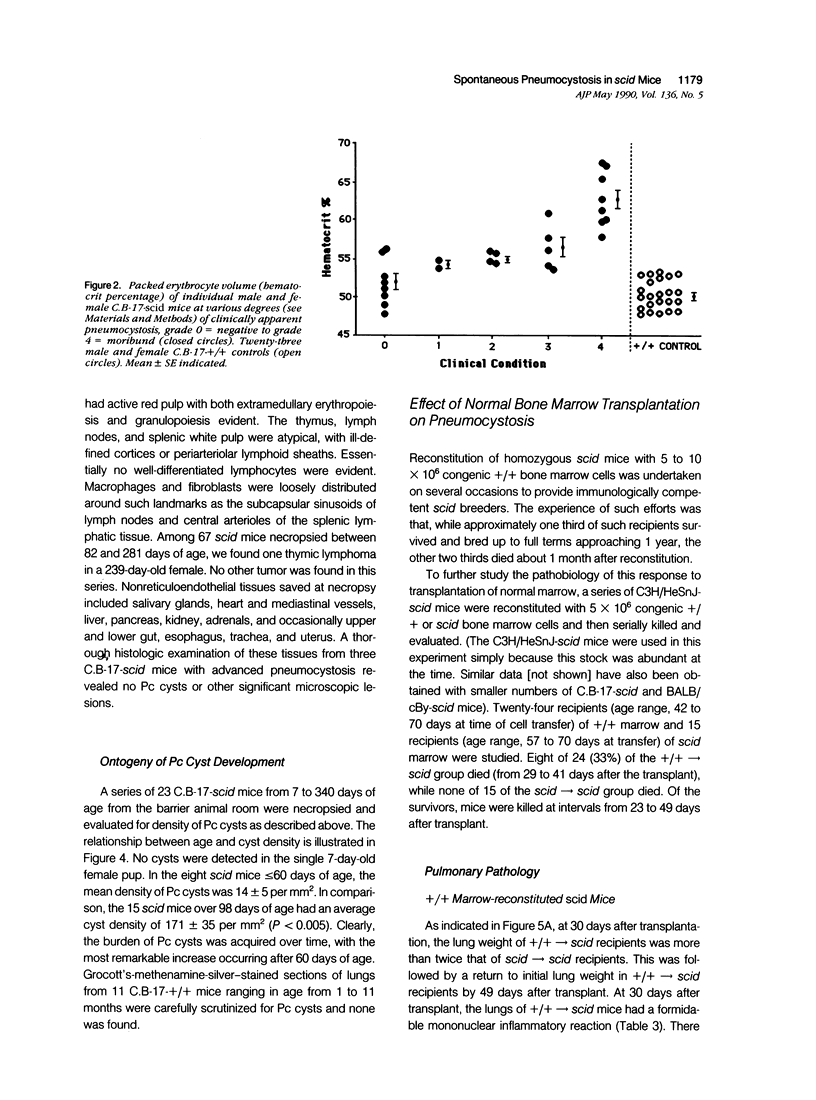
Abstract
The opportunistic pathogen Pneumocystis carinii (Pc) poses a major clinical health problem in individuals with immune deficiency, including those patients with human immunodeficiency (HIV)-associated acquired immune deficiency disease (AIDS). Heretofore, in vivo investigations of the biology of Pc and pathogenesis of pneumocystosis have generally employed steroid-induced immune suppression with antibiotic prophylaxis and protein deprivation. This approach has many drawbacks, chief among them being the widespread, multiple interacting effects caused by the inducing agents. Athymic (nude) mice and rats have been used, but are less than ideal, as the immune defect primarily affects T lymphocytes. This article describes the natural history, pathobiology, and environmental effects on Pc pneumonitis in nonaxenically housed mice homozygous for the autosomal recessive mutation 'severe combined immunodeficiency' (scid), which almost totally lack both cell-mediated and antibody-mediated immune functions. The predictability, unequivocal expression, high morbidity, and well-defined genetic basis make scid/scid mutant mice the model of choice for in vivo studies of spontaneous pneumocystosis.
Full text
PDF



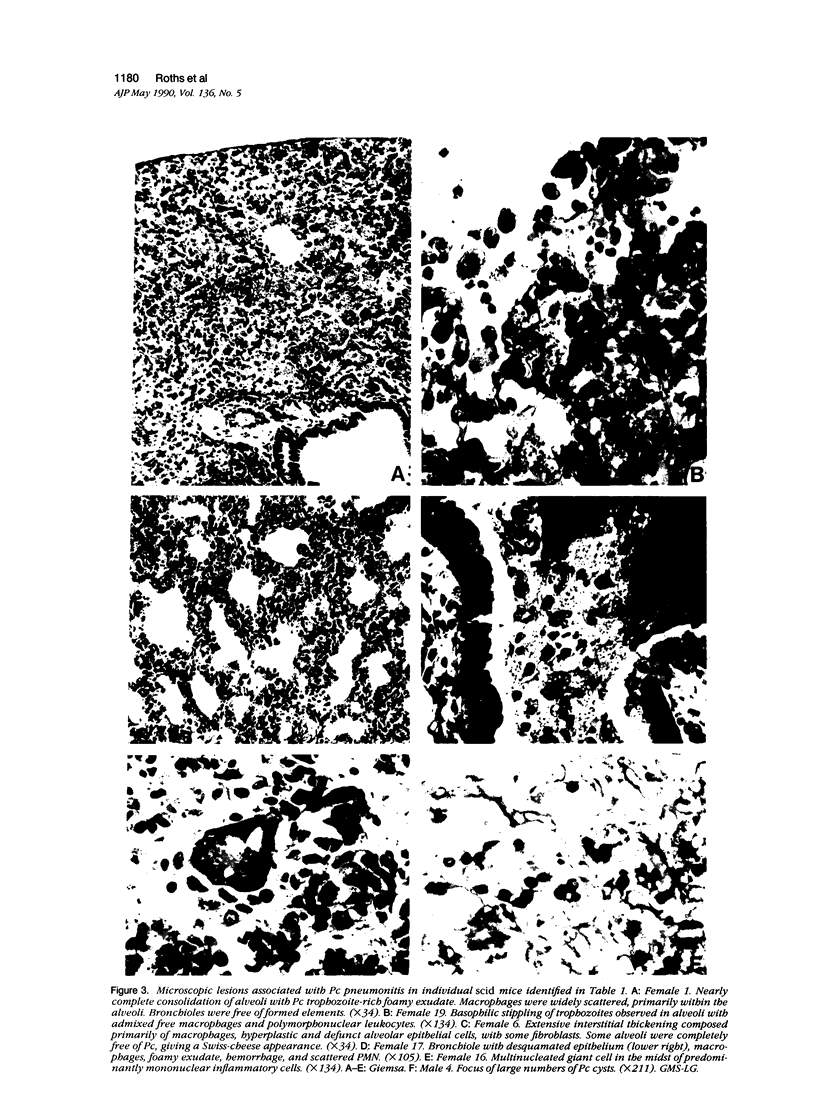
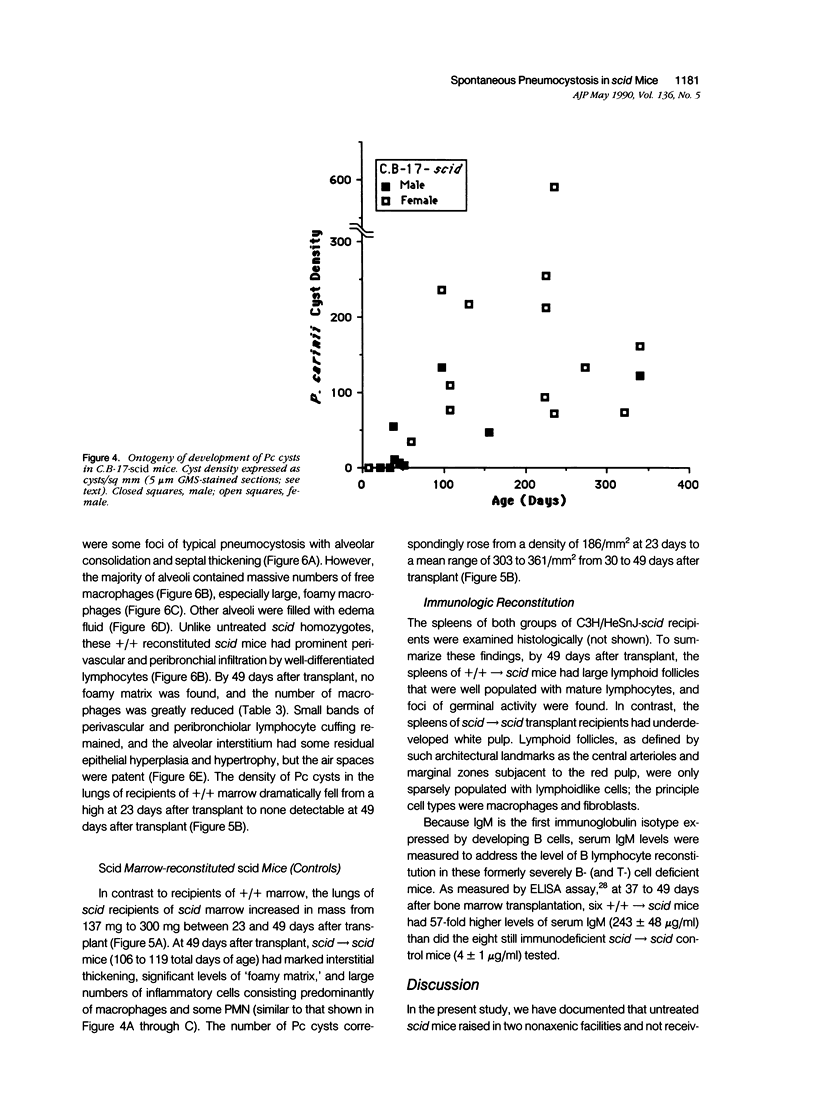
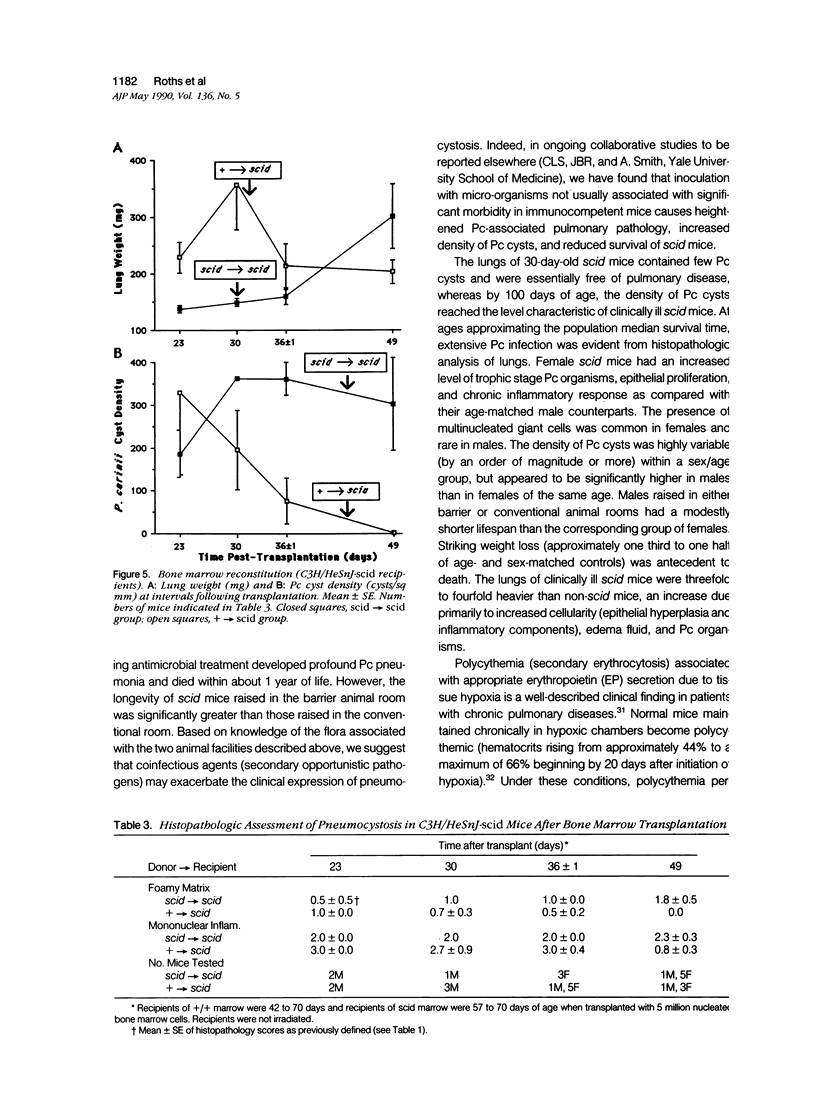
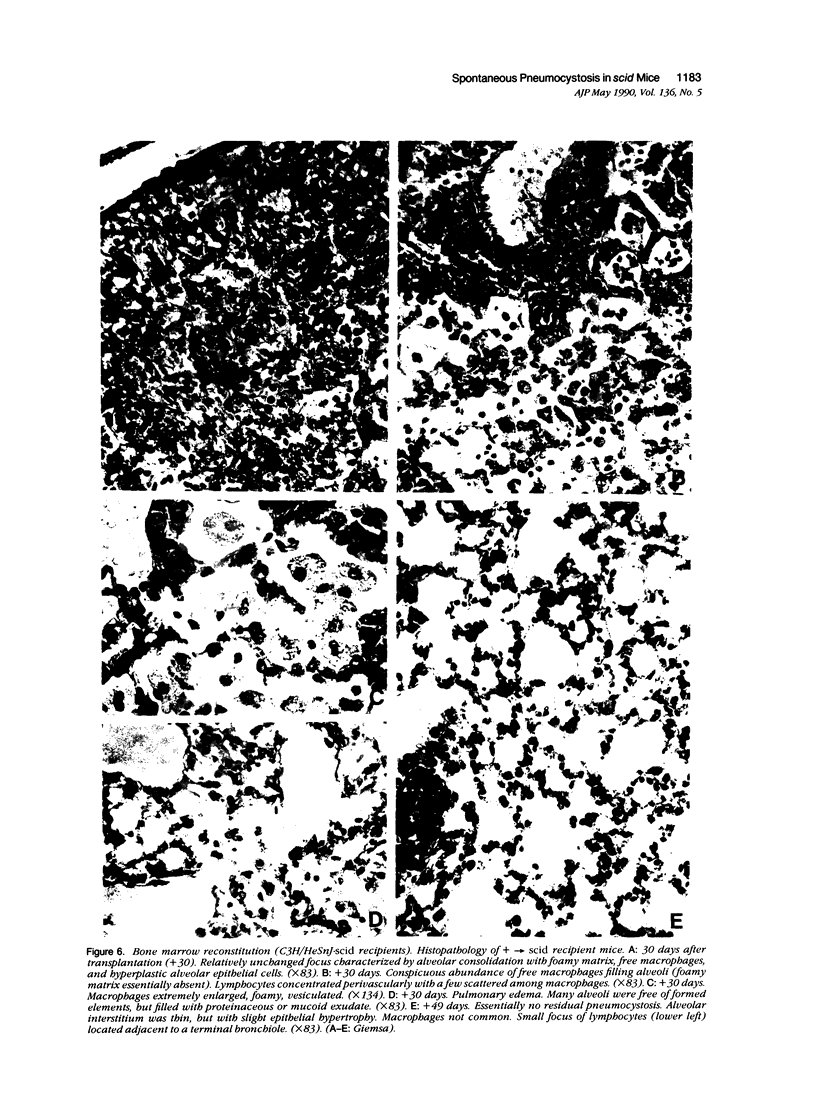



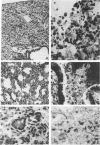
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Bosma M. J., Bosma G. C., Unanue E. R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986 Jul 1;137(1):4–9. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Davisson M. T., Ruetsch N. R., Sweet H. O., Shultz L. D., Bosma M. J. The mouse mutation severe combined immune deficiency (scid) is on chromosome 16. Immunogenetics. 1989;29(1):54–57. doi: 10.1007/BF02341614. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Czitrom A. A., Edwards S., Phillips R. A., Bosma M. J., Marrack P., Kappler J. W. The function of antigen-presenting cells in mice with severe combined immunodeficiency. J Immunol. 1985 Apr;134(4):2276–2280. [PubMed] [Google Scholar]
- Deschryver-Kecskemeti K., Bancroft G. J., Bosma G. C., Bosma M. J., Unanue E. R. Pathology of Listeria infection in murine severe combined immunodeficiency. A study by immunohistochemistry and electron microscopy. Lab Invest. 1988 Jun;58(6):698–705. [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C. Monocyte-mediated serum-independent damage to hyphal and pseudohyphal forms of Candida albicans in vitro. J Clin Invest. 1981 Jan;67(1):173–182. doi: 10.1172/JCI110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Dorshkind K., Pollack S. B., Bosma M. J., Phillips R. A. Natural killer (NK) cells are present in mice with severe combined immunodeficiency (scid). J Immunol. 1985 Jun;134(6):3798–3801. [PubMed] [Google Scholar]
- Edman J. C., Kovacs J. A., Masur H., Santi D. V., Elwood H. J., Sogin M. L. Ribosomal RNA sequence shows Pneumocystis carinii to be a member of the fungi. Nature. 1988 Aug 11;334(6182):519–522. doi: 10.1038/334519a0. [DOI] [PubMed] [Google Scholar]
- Elin R. J., Edelin J. B., Wolff S. M. Infection and immunoglobulin concentrations in Chediak-Higashi mice. Infect Immun. 1974 Jul;10(1):88–91. doi: 10.1128/iai.10.1.88-91.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel J. K., Good J. T., Shultz J. A. Latent Pneumocystis infection of rats, relapse, and chemotherapy. Lab Invest. 1966 Oct;15(10):1559–1577. [PubMed] [Google Scholar]
- Fulop G. M., Bosma G. C., Bosma M. J., Phillips R. A. Early B-cell precursors in scid mice: normal numbers of cells transformable with Abelson murine leukemia virus (A-MuLV). Cell Immunol. 1988 Apr 15;113(1):192–201. doi: 10.1016/0008-8749(88)90017-2. [DOI] [PubMed] [Google Scholar]
- Furuta T., Ueda K., Fujiwara K. Experimental Pneumocystis carinii infection in nude rats. Jpn J Exp Med. 1984 Apr;54(2):65–72. [PubMed] [Google Scholar]
- GALLO R. C., FRAIMOW W., CATHCART R. T., ERSLEV A. J. ERYTHROPOIETIC RESPONSE IN CHRONIC PULMONARY DISEASE. Arch Intern Med. 1964 Apr;113:559–568. doi: 10.1001/archinte.1964.00280100067011. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., Feldman S., Aur R. J., Verzosa M. S., Hustu H. O., Simone J. V. Intensity of immunosuppressive therapy and the incidence of Pneumocystis carinii pneumonitis. Cancer. 1975 Dec;36(6):2004–2009. doi: 10.1002/cncr.2820360912. [DOI] [PubMed] [Google Scholar]
- Hughes W. T. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J Infect Dis. 1982 Jun;145(6):842–848. doi: 10.1093/infdis/145.6.842. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., Price R. A., Kim H. K., Coburn T. P., Grigsby D., Feldman S. Pneumocystis carinii pneumonitis in children with malignancies. J Pediatr. 1973 Mar;82(3):404–415. doi: 10.1016/s0022-3476(73)80113-1. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., Price R. A., Sisko F., Havron W. S., Kafatos A. G., Schonland M., Smythe P. M. Protein-calorie malnutrition. A host determinant for Pneumocystis carinii infection. Am J Dis Child. 1974 Jul;128(1):44–52. doi: 10.1001/archpedi.1974.02110260046008. [DOI] [PubMed] [Google Scholar]
- Keighley G. H., Lowy P., Russel E. S., Thompson M. W. Analysis of erythroid homeostatic mechanisms in normal and genetically anaemic mice. Br J Haematol. 1966 Jul;12(4):461–477. doi: 10.1111/j.1365-2141.1966.tb05655.x. [DOI] [PubMed] [Google Scholar]
- Lauzon R. J., Siminovitch K. A., Fulop G. M., Phillips R. A., Roder J. C. An expanded population of natural killer cells in mice with severe combined immunodeficiency (SCID) lack rearrangement and expression of T cell receptor genes. J Exp Med. 1986 Nov 1;164(5):1797–1802. doi: 10.1084/jem.164.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S., Greenfield R. A., Joyce W. A., Kincade P. W. Inoculation candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1988 Dec;56(12):3162–3166. doi: 10.1128/iai.56.12.3162-3166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- Meuwissen J. H., Tauber I., Leeuwenberg A. D., Beckers P. J., Sieben M. Parasitologic and serologic observations of infection with Pneumocystis in humans. J Infect Dis. 1977 Jul;136(1):43–49. doi: 10.1093/infdis/136.1.43. [DOI] [PubMed] [Google Scholar]
- Mills J. Pneumocystis carinii and Toxoplasma gondii infections in patients with AIDS. Rev Infect Dis. 1986 Nov-Dec;8(6):1001–1011. doi: 10.1093/clinids/8.6.1001. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Pifer L. L., Hughes W. T., Murphy M. J., Jr Propagation of Pneumocystis carinii in vitro. Pediatr Res. 1977 Apr;11(4):305–316. doi: 10.1203/00006450-197704000-00010. [DOI] [PubMed] [Google Scholar]
- Richter C. B., Thigpen J. E., Richter C. S., Mackenzie J. M., Jr Fatal pneumonia with terminal emaciation in nude mice caused by pneumonia virus of mice. Lab Anim Sci. 1988 Jun;38(3):255–261. [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Shultz L. D., Hardy R. R., Hayakawa K., Herzenberg L. A. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 1986 Jun 13;232(4756):1423–1425. doi: 10.1126/science.3487115. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Benacerraf B. Immunologic events in experimental hypersensitivity granulomas. Am J Pathol. 1973 Jun;71(3):349–364. [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Linke M. J., Pogue C. L., Huerkamp M. J., Chrisp C. E., Lerro A. V., Wixson S. K., Hall E., Shultz L. D. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect Immun. 1989 Jan;57(1):62–70. doi: 10.1128/iai.57.1.62-70.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Linke M. J. A comparison of the antigenic characteristics of rat and human Pneumocystis carinii by immunoblotting. J Immunol. 1987 Apr 1;138(7):2257–2265. [PubMed] [Google Scholar]
- Walzer P. D., Powell R. D., Jr, Yoneda K., Rutledge M. E., Milder J. E. Growth characteristics and pathogenesis of experimental Pneumocystis carinii pneumonia. Infect Immun. 1980 Mar;27(3):928–937. doi: 10.1128/iai.27.3.928-937.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., Schnelle V., Armstrong D., Rosen P. P. Nude mouse: a new experimental model for Pneumocystis carinii infection. Science. 1977 Jul 8;197(4299):177–179. doi: 10.1126/science.301657. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Schultz M. G., Western K. A., Robbins J. B. Pneumocystis carinii pneumonia and primary immune deficiency diseases of infancy and childhood. J Pediatr. 1973 Mar;82(3):416–422. doi: 10.1016/s0022-3476(73)80114-3. [DOI] [PubMed] [Google Scholar]