Abstract
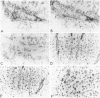
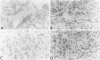
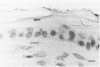
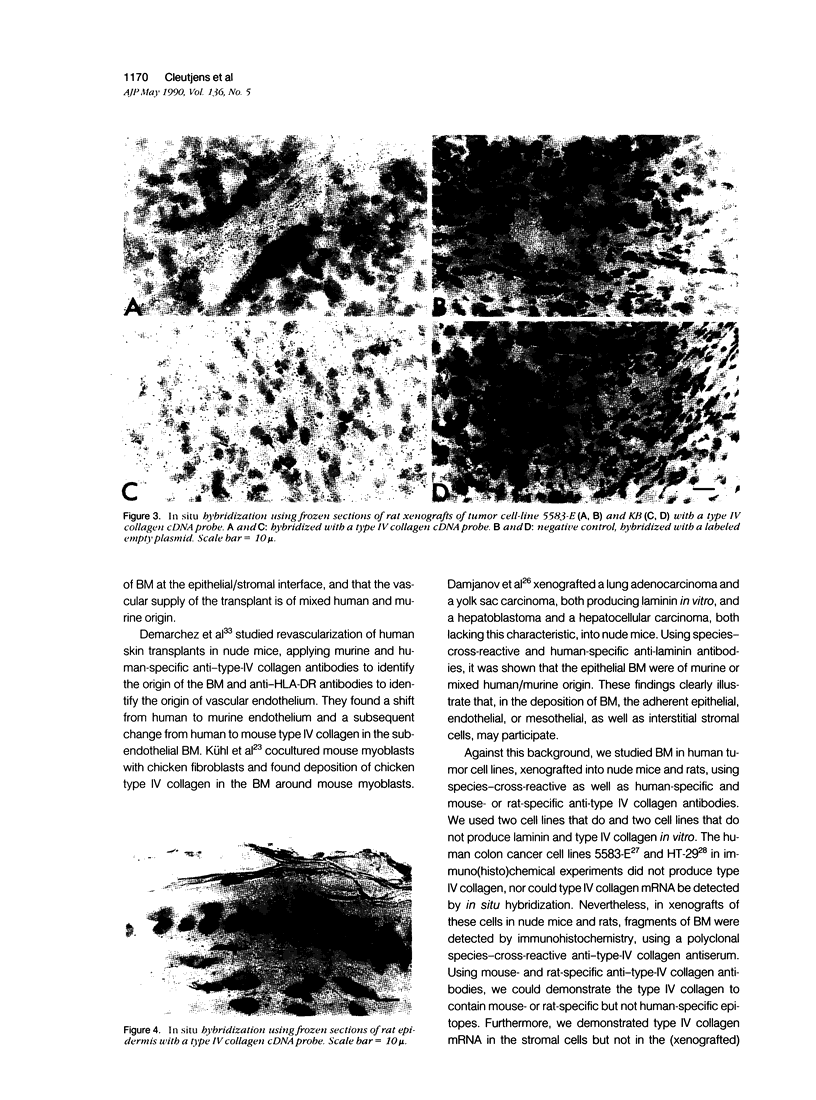
The origin of basement membrane (BM), deposited in epithelial neoplasms, was studied in xenografts of human tumor cell lines in nude mice and rats. Cell lines were chosen that in vitro do (WISH, KB) or do not (5583-E; HT-29) produce BM components, more specifically, type IV collagen. Basement membrane deposition was studied by immunohistochemistry, using species cross-reactive polyclonal anti-human type IV collagen antiserum, mouse- and rat-specific polyclonal anti-mouse type IV collagen antiserum, human-specific monoclonal anti-type-IV collagen antibody, and by in situ hybridization, using a 32S-labeled species cross-reactive cDNA probe, specific for type IV collagen mRNA. In the xenografts, species-cross-reactive anti-type-IV collagen antiserum demonstrated the presence of irregular and discontinuous BM. In 5583-E and HT-29 xenografts, only murine type IV collagen epitopes were detected. In contrast, in WISH and in KB xenografts, the BM stained human as well as murine type IV collagen epitopes. By in situ hybridization, type IV collagen mRNA was detectable in stromal cells only in 5583-E and HT-29 xenografts, but in both epithelial and stromal cells in WISH and KB xenografts. These results indicate that in this model system epithelial tumor cells and stromal (mesenchymal) cells are involved in the production and deposition of a BM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Vaheri A., Krieg T., Timpl R. Biosynthesis of two subunits of type IV procollagen and of other basement membrane proteins by a human tumor cell line. Eur J Biochem. 1980 Aug;109(1):247–255. doi: 10.1111/j.1432-1033.1980.tb04790.x. [DOI] [PubMed] [Google Scholar]
- Banerjee S. D., Cohn R. H., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Production by the epithelium and role in maintaining lobular morphology. J Cell Biol. 1977 May;73(2):445–463. doi: 10.1083/jcb.73.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell A. G., Bessem C. C., Slavkin H. C. Possible functions of mesenchyme cell-derived fibronectin during formation of basal lamina. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3711–3715. doi: 10.1073/pnas.78.6.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtin P., Chavanel G., Foidart J. M., Martin E. Antigens of the basement membrane and the peritumoral stroma in human colonic adenocarcinomas: an immunofluorescence study. Int J Cancer. 1982 Jul 15;30(1):13–20. doi: 10.1002/ijc.2910300104. [DOI] [PubMed] [Google Scholar]
- Damjanov I., Damjanov N., Knowles B. B., Engvall E. Origin of laminin in the extracellular matrix of human tumor xenografts in nude mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(1):45–52. doi: 10.1007/BF02912083. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G., Bernfield M. Type I collagen reduces the degradation of basal lamina proteoglycan by mammary epithelial cells. J Cell Biol. 1981 Oct;91(1):281–286. doi: 10.1083/jcb.91.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchez M., Hartmann D. J., Prunieras M. An immunohistological study of the revascularization process in human skin transplanted onto the nude mouse. Transplantation. 1987 Jun;43(6):896–903. [PubMed] [Google Scholar]
- Dodson J. W., Hay E. D. Secretion of collagenous stroma by isolated epithelium grown in vitro. Exp Cell Res. 1971 Mar;65(1):215–220. doi: 10.1016/s0014-4827(71)80069-1. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Bere E. W., Jr, Yaar M., Rennard S. I., Gullino M., Martin G. R., Katz S. I. Distribution and immunoelectron microscopic localization of laminin, a noncollagenous basement membrane glycoprotein. Lab Invest. 1980 Mar;42(3):336–342. [PubMed] [Google Scholar]
- Forster S. J., Talbot I. C., Critchley D. R. Laminin and fibronectin in rectal adenocarcinoma: relationship to tumour grade, stage and metastasis. Br J Cancer. 1984 Jul;50(1):51–61. doi: 10.1038/bjc.1984.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbi C., Wollman S. H. Basal lamina formation on thyroid epithelia in separated follicles in suspension culture. J Cell Biol. 1982 Aug;94(2):489–492. doi: 10.1083/jcb.94.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould V. E., Battifora H. Origin and significance of the basal lamina and some interstitial fibrillar components in epithelial neoplasms. Pathol Annu. 1976;11:353–386. [PubMed] [Google Scholar]
- HAYFLICK L. The establishment of a line (WISH) of human amnion cells in continuous cultivation. Exp Cell Res. 1961 Feb;23:14–20. doi: 10.1016/0014-4827(61)90059-3. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Robey P. G., Barrach H. J., Wilczek J., Rennard S. I., Martin G. R. Isolation of a heparan sulfate-containing proteoglycan from basement membrane. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4494–4498. doi: 10.1073/pnas.77.8.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenith M. G., Cleutjens J. P., Beek C., vd Linden E., De Goeij A. F., Bosman F. T. Human specific anti-type IV collagen monoclonal antibodies, characterization and immunohistochemical application. Histochemistry. 1987;87(2):123–128. doi: 10.1007/BF00533396. [DOI] [PubMed] [Google Scholar]
- Heathcote J. G., Bruns R. R., Orkin R. W. Biosynthesis of sulphated macromolecules by rabbit lens epithelium. II. Relationship to basement membrane formation. J Cell Biol. 1984 Sep;99(3):861–869. doi: 10.1083/jcb.99.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung J., Bohnert A., Phan-Than L., Krieg T., Fusenig N. E. Basement membrane formation by malignant mouse keratinocyte cell lines in organotypic culture and transplants: correlation with degree of morphologic differentiation. J Cancer Res Clin Oncol. 1987;113(4):325–341. doi: 10.1007/BF00397716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallioinen M., Autio-Harmainen H., Dammert K., Risteli J., Risteli L. Discontinuity of the basement membrane in fibrosing basocellular carcinomas and basosquamous carcinomas of the skin: an immunohistochemical study with human laminin and type IV collagen antibodies. J Invest Dermatol. 1984 Mar;82(3):248–251. doi: 10.1111/1523-1747.ep12260190. [DOI] [PubMed] [Google Scholar]
- Kefalides N. A., Alper R., Clark C. C. Biochemistry and metabolism of basement membranes. Int Rev Cytol. 1979;61:167–228. doi: 10.1016/s0074-7696(08)61998-1. [DOI] [PubMed] [Google Scholar]
- Kühl U., Ocalan M., Timpl R., Mayne R., Hay E., von der Mark K. Role of muscle fibroblasts in the deposition of type-IV collagen in the basal lamina of myotubes. Differentiation. 1984;28(2):164–172. doi: 10.1111/j.1432-0436.1984.tb00279.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Lipper S., Kahn L. B., Reddick R. L. The myofibroblast. Pathol Annu. 1980;15(Pt 1):409–441. [PubMed] [Google Scholar]
- McArdle J. P., Müller H. K., Roff B. T., Murphy W. H. Basal lamina redevelopment in tumours metastatic to brain:an immunoperoxidase study using an antibody to type-IV collagen. Int J Cancer. 1984 Nov 15;34(5):633–638. doi: 10.1002/ijc.2910340508. [DOI] [PubMed] [Google Scholar]
- Nusgens B., Merrill C., Lapiere C., Bell E. Collagen biosynthesis by cells in a tissue equivalent matrix in vitro. Coll Relat Res. 1984 Oct;4(5):351–363. doi: 10.1016/s0174-173x(84)80003-5. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Tryggvason K., Myers J. C., Kurkinen M., Lebo R., Cheung M. C., Prockop D. J., Boyd C. D. cDNA clones coding for the pro-alpha1(IV) chain of human type IV procollagen reveal an unusual homology of amino acid sequences in two halves of the carboxyl-terminal domain. J Biol Chem. 1985 Jun 25;260(12):7681–7687. [PubMed] [Google Scholar]
- Sanderson R. D., Fitch J. M., Linsenmayer T. R., Mayne R. Fibroblasts promote the formation of a continuous basal lamina during myogenesis in vitro. J Cell Biol. 1986 Mar;102(3):740–747. doi: 10.1083/jcb.102.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa S., Modesti A., Triche T. J. Extracellular matrix synthesis by undifferentiated childhood tumor cell lines. Am J Pathol. 1987 Oct;129(1):74–85. [PMC free article] [PubMed] [Google Scholar]
- Seemayer T. A., Lagacé R., Schürch W., Thelmo W. L. The myofibroblast: biologic, pathologic, and theoretical considerations. Pathol Annu. 1980;15(Pt 1):443–470. [PubMed] [Google Scholar]
- Simon-Assmann P., Bouziges F., Arnold C., Haffen K., Kedinger M. Epithelial-mesenchymal interactions in the production of basement membrane components in the gut. Development. 1988 Feb;102(2):339–347. doi: 10.1242/dev.102.2.339. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Williams J. E., Liotta L. A., Martin G. R. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984 Nov 23;226(4677):982–985. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstijnen C. P., Arends J. W., Moerkerk P. T., Geraedts J. P., Sekikawa K., Uitendaal M. P., Bosman F. T. The establishment and characterization of two new cell lines derived from a single human colonic adenocarcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;53(4):191–197. doi: 10.1007/BF02890243. [DOI] [PubMed] [Google Scholar]
- Wewer U., Albrechtsen R., Manthorpe M., Varon S., Engvall E., Ruoslahti E. Human laminin isolated in a nearly intact, biologically active form from placenta by limited proteolysis. J Biol Chem. 1983 Oct 25;258(20):12654–12660. [PubMed] [Google Scholar]