Abstract
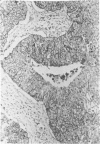
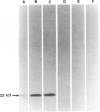
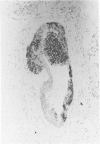
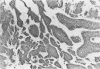
After immunization of mice with viable cells of a metastasis of a laryngeal squamous cell carcinoma, a monoclonal antibody E 48 was obtained that detects an epitope present exclusively in squamous and transitional epithelium and their neoplastic counterparts. Immunoblotting revealed that E 48 recognizes a 22 kd molecule. Seventy-five of 76 squamous cell carcinomas from the head and neck, lung, cervix, and skin stained positively, whereas various adenocarcinomas from the colon, lung, and breast, and small cell lung carcinomas consistently stained negatively. The E 48 antigen, which is formaldehyde resistant, appears to be a reliable marker for differentiation of squamous cell carcinomas from adenocarcinoma, and small cell carcinomas.
Full text
PDF

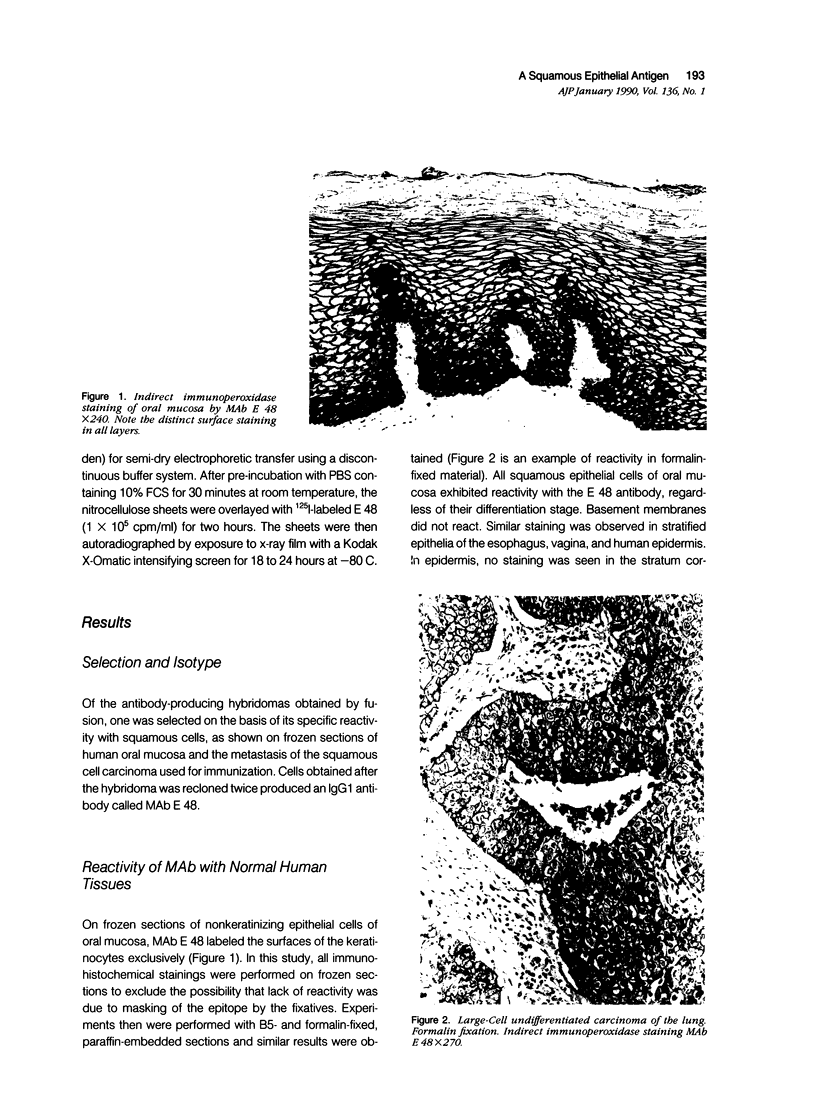
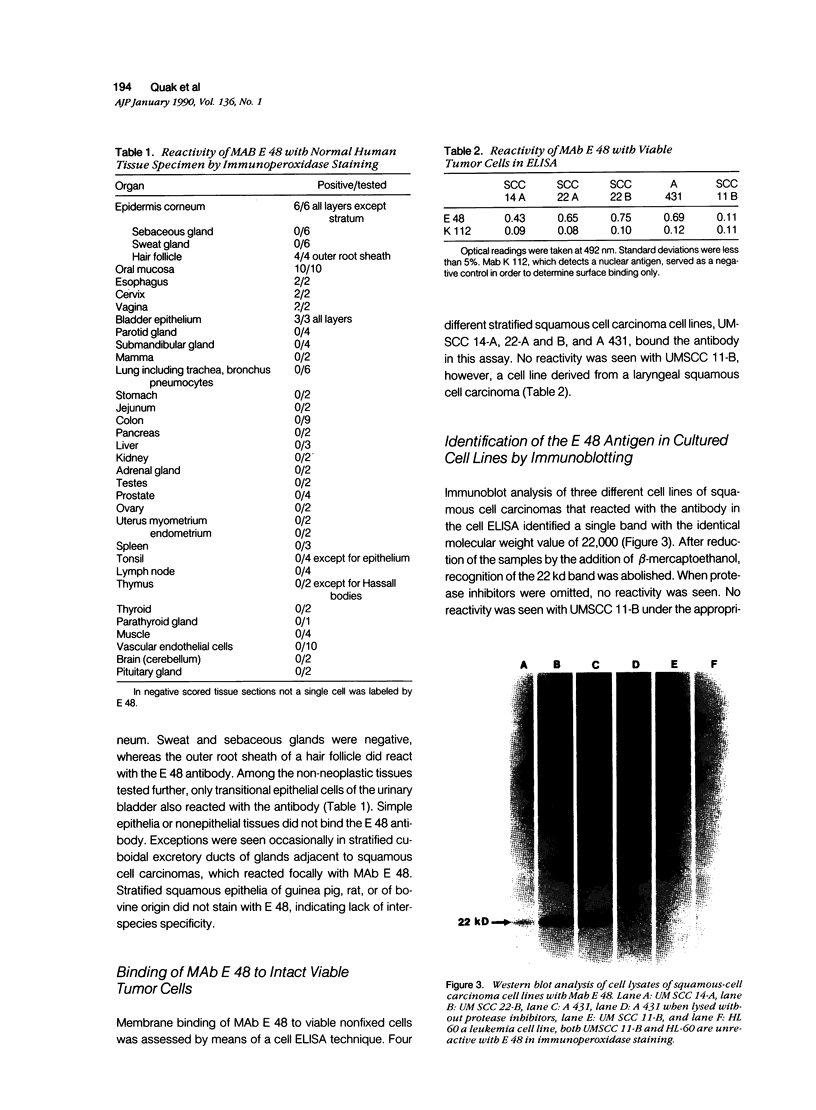



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Haisma H. J., Hilgers J., Zurawski V. R., Jr Iodination of monoclonal antibodies for diagnosis and radiotherapy using a convenient one vial method. J Nucl Med. 1986 Dec;27(12):1890–1895. [PubMed] [Google Scholar]
- Hammond M. E., Sause W. T. Large cell neuroendocrine tumors of the lung. Clinical significance and histopathologic definition. Cancer. 1985 Oct 1;56(7):1624–1629. doi: 10.1002/1097-0142(19851001)56:7<1624::aid-cncr2820560727>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hess F. G., Jr, McDowell E. M., Trump B. F. Pulmonary cytology: current status of cytologic typing of respiratory tract tumors. Am J Pathol. 1981 May;103(2):323–333. [PMC free article] [PubMed] [Google Scholar]
- Hirsch F. R., Matthews M. J., Aisner S., Campobasso O., Elema J. D., Gazdar A. F., Mackay B., Nasiell M., Shimosato Y., Steele R. H. Histopathologic classification of small cell lung cancer. Changing concepts and terminology. Cancer. 1988 Sep 1;62(5):973–977. doi: 10.1002/1097-0142(19880901)62:5<973::aid-cncr2820620521>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Peters L. C., Brandhorst J. S., Hanna M. G., Jr Preparation of immunotherapeutic autologous tumor cell vaccines from solid tumors. Cancer Res. 1979 Apr;39(4):1353–1360. [PubMed] [Google Scholar]
- Quak J. J., Balm A. J., Brakkee J. G., Scheper R. J., Haisma H. J., Braakhuis B. J., Meijer C. J., Snow G. B. Localization and imaging of radiolabelled monoclonal antibody against squamous-cell carcinoma of the head and neck in tumor-bearing nude mice. Int J Cancer. 1989 Sep 15;44(3):534–538. doi: 10.1002/ijc.2910440327. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Said J. W., Nash G., Sassoon A. F., Shintaku I. P., Banks-Schlegel S. Involucrin in lung tumors. A specific marker for squamous differentiation. Lab Invest. 1983 Nov;49(5):563–568. [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Spitz M., Spitz L., Thorpe R., Eugui E. Intrasplenic primary immunization for the production of monoclonal antibodies. J Immunol Methods. 1984 May 11;70(1):39–43. doi: 10.1016/0022-1759(84)90387-9. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]