Abstract
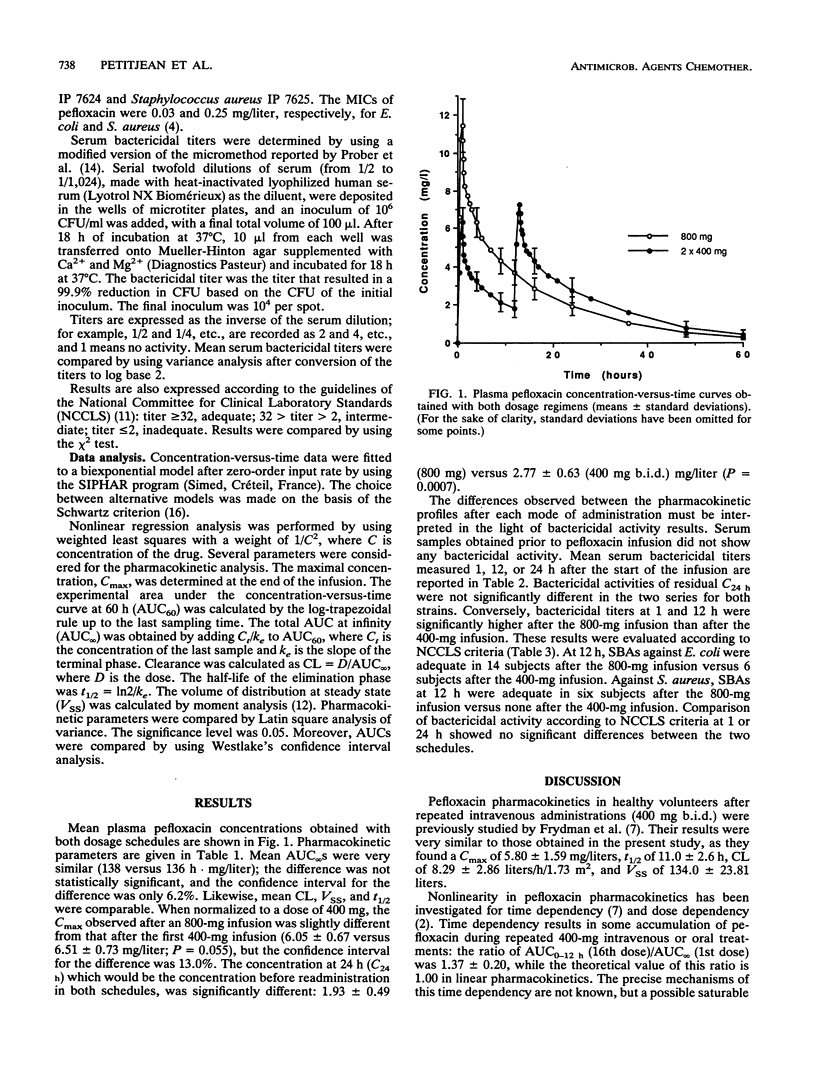
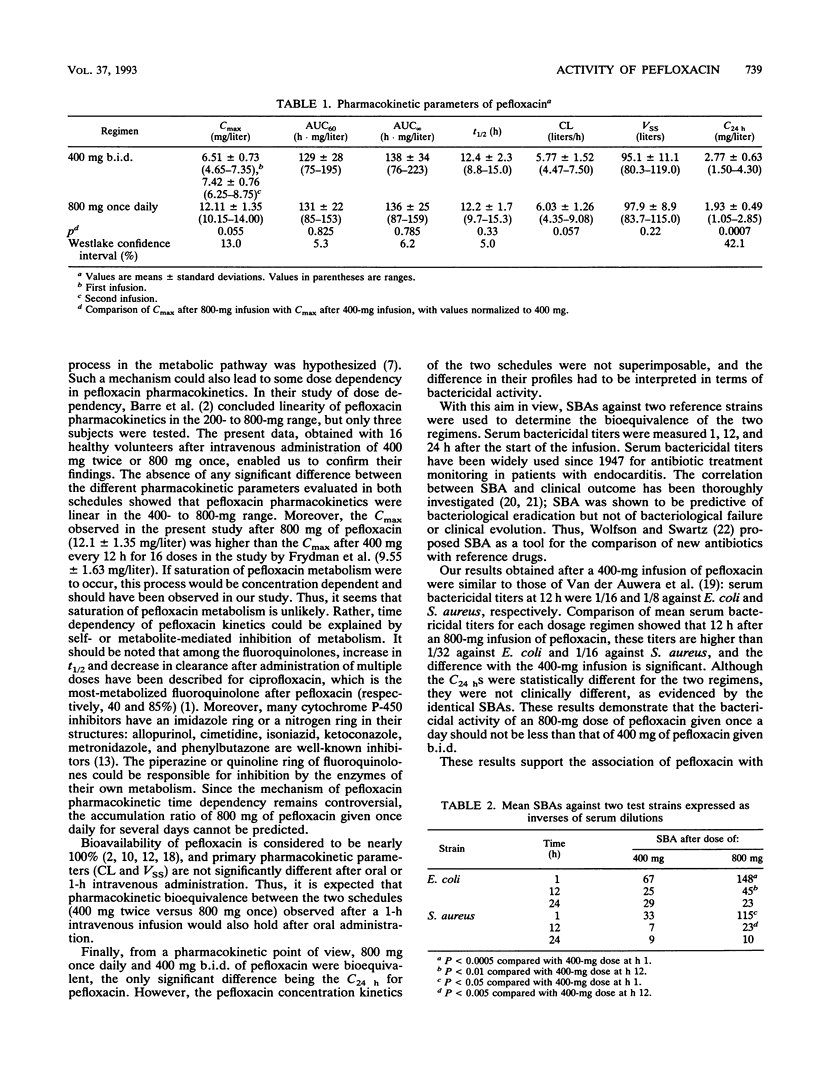
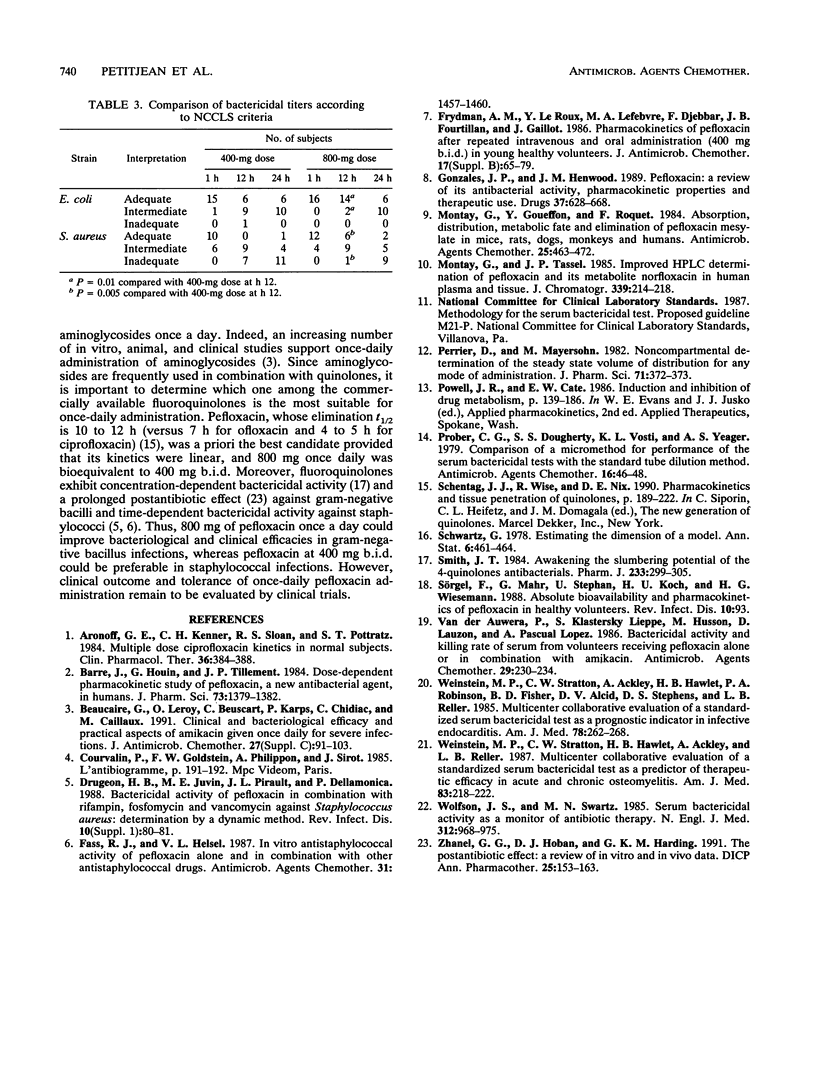
Pefloxacin pharmacokinetics and serum bactericidal activities (SBA) against Escherichia coli and Staphylococcus aureus were compared after intravenous infusion of either a single 800-mg dose or twice-daily 400-mg doses into 16 healthy volunteers. Plasma pefloxacin concentrations were measured for up to 60 h, and SBAs were determined 1, 12, and 24 h after the start of the infusion. The mean areas under the concentration-versus-time curve for plasma were not different (138 versus 136 h.mg/liter). The mean clearances, volumes of distribution, and half-lives were also comparable. The mean (+/- standard deviation) maximal concentration after the 800-mg infusion was 12.11 +/- 1.35 versus 6.51 +/- 0.73 mg/liter after the first 400-mg infusion and 7.42 +/- 0.76 mg/liter after the second 400-mg infusion. Mean trough concentrations at 24 h were significantly different: 2.77 +/- 0.63 (800 mg) versus 1.93 +/- 0.49 (400 mg twice) mg/liter (P = 0.0007). Mean SBAs against E. coli after 800 mg of pefloxacin were higher than 1/128 (1 h), 1/32 (12 h), and 1/16 (24 h). Mean SBAs against S. aureus under the same conditions were higher than 1/64 (1 h), 1/16 (12 h), and 1/8 (24 h). Mean SBAs at 1 and 12 h were significantly higher after the 800-mg infusion than after the 400-mg infusion but were similar at 24 h for both regimens. Comparison of SBAs according to National Committee for Clinical Laboratory Standards criteria showed a similar adequacy at 24 h for both regimens against both strains. Administration of 800 mg of pefloxacin once a day is bioequivalent to 400 mg twice a day, and bactericidal activity of the 800-mg infusion is not less than that of two 400-mg infusions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff G. E., Kenner C. H., Sloan R. S., Pottratz S. T. Multiple-dose ciprofloxacin kinetics in normal subjects. Clin Pharmacol Ther. 1984 Sep;36(3):384–388. doi: 10.1038/clpt.1984.192. [DOI] [PubMed] [Google Scholar]
- Barre J., Houin G., Tillement J. P. Dose-dependent pharmacokinetic study of pefloxacin, a new antibacterial agent, in humans. J Pharm Sci. 1984 Oct;73(10):1379–1382. doi: 10.1002/jps.2600731014. [DOI] [PubMed] [Google Scholar]
- Beaucaire G., Leroy O., Beuscart C., Karp P., Chidiac C., Caillaux M. Clinical and bacteriological efficacy, and practical aspects of amikacin given once daily for severe infections. J Antimicrob Chemother. 1991 May;27 (Suppl 100):91–103. doi: 10.1093/jac/27.suppl_c.91. [DOI] [PubMed] [Google Scholar]
- Fass R. J., Helsel V. L. In vitro antistaphylococcal activity of pefloxacin alone and in combination with other antistaphylococcal drugs. Antimicrob Agents Chemother. 1987 Oct;31(10):1457–1460. doi: 10.1128/aac.31.10.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman A. M., Le Roux Y., Lefebvre M. A., Djebbar F., Fourtillan J. B., Gaillot J. Pharmacokinetics of pefloxacin after repeated intravenous and oral administration (400 mg bid) in young healthy volunteers. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):65–79. doi: 10.1093/jac/17.suppl_b.65. [DOI] [PubMed] [Google Scholar]
- Gonzalez J. P., Henwood J. M. Pefloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1989 May;37(5):628–668. doi: 10.2165/00003495-198937050-00003. [DOI] [PubMed] [Google Scholar]
- Montay G., Goueffon Y., Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984 Apr;25(4):463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montay G., Tassel J. P. Improved high-performance liquid chromatographic determination of pefloxacin and its metabolite norfloxacin in human plasma and tissue. J Chromatogr. 1985 Apr 12;339(1):214–218. doi: 10.1016/s0378-4347(00)84647-2. [DOI] [PubMed] [Google Scholar]
- Perrier D., Mayersohn M. Noncompartmental determination of the steady-state volume of distribution for any mode of administration. J Pharm Sci. 1982 Mar;71(3):372–373. doi: 10.1002/jps.2600710332. [DOI] [PubMed] [Google Scholar]
- Prober C. G., Dougherty S. S., Vosti K. L., Yeager A. S. Comparison of a micromethod for performance of the serum bactericidal test with the standard tube dilution method. Antimicrob Agents Chemother. 1979 Jul;16(1):46–48. doi: 10.1128/aac.16.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Klastersky J., Lieppe S., Husson M., Lauzon D., Lopez A. P. Bactericidal activity and killing rate of serum from volunteers receiving pefloxacin alone or in combination with amikacin. Antimicrob Agents Chemother. 1986 Feb;29(2):230–234. doi: 10.1128/aac.29.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Stratton C. W., Ackley A., Hawley H. B., Robinson P. A., Fisher B. D., Alcid D. V., Stephens D. S., Reller L. B. Multicenter collaborative evaluation of a standardized serum bactericidal test as a prognostic indicator in infective endocarditis. Am J Med. 1985 Feb;78(2):262–269. doi: 10.1016/0002-9343(85)90436-x. [DOI] [PubMed] [Google Scholar]
- Weinstein M. P., Stratton C. W., Hawley H. B., Ackley A., Reller L. B. Multicenter collaborative evaluation of a standardized serum bactericidal test as a predictor of therapeutic efficacy in acute and chronic osteomyelitis. Am J Med. 1987 Aug;83(2):218–222. doi: 10.1016/0002-9343(87)90688-7. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Swartz M. N. Drug therapy. Serum bactericidal activity as a monitor of antibiotic therapy. N Engl J Med. 1985 Apr 11;312(15):968–975. doi: 10.1056/NEJM198504113121507. [DOI] [PubMed] [Google Scholar]
- Zhanel G. G., Hoban D. J., Harding G. K. The postantibiotic effect: a review of in vitro and in vivo data. DICP. 1991 Feb;25(2):153–163. doi: 10.1177/106002809102500210. [DOI] [PubMed] [Google Scholar]



