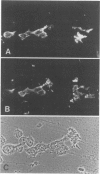
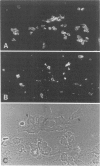
Abstract
Vimentin expression, growth fractions (GF), and estrogen receptor (ER) levels were determined for 90 untreated primary breast carcinomas. Coexpression of keratin and vimentin was found in approximately 20% of the tumors regardless of menopausal status. Vimentin was expressed preferentially in tumor cells of high-grade ductal breast carcinomas (15 of 28 histologic grade 3 vs. 0 of 40 grades 1 and 2). Vimentin expression was found preferentially in tumors with high GF (greater than 15% Ki-67 positive by immunoperoxidase staining) and low ER levels (less than 60 fmols/mg protein by a monoclonal enzyme immunoassay). Sixty-eight percent of tumors in this group were vimentin positive and 88% of all vimentin-positive tumors fell into this category. More than 50% of the tumor cells coexpressed vimentin and keratin. Thus, vimentin expression may be helpful in identifying a substantial subset of ER-independent breast carcinomas with poor prognostic indicators.
Full text
PDF
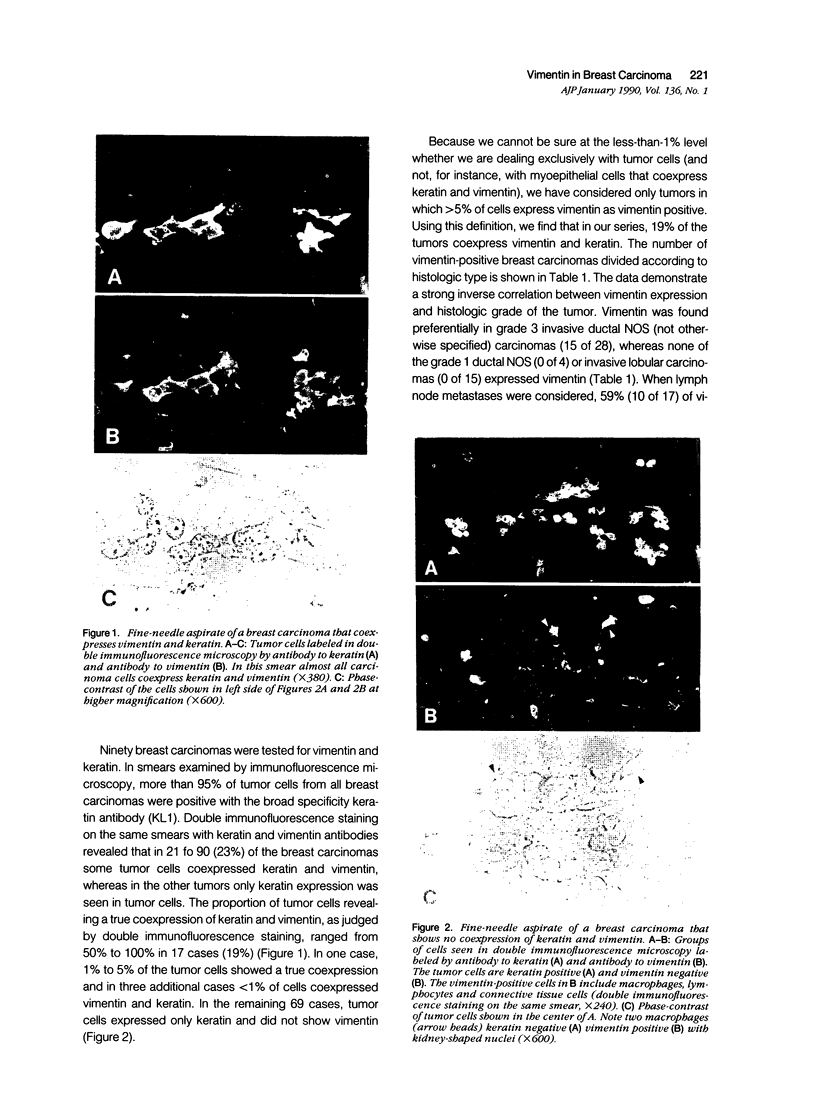

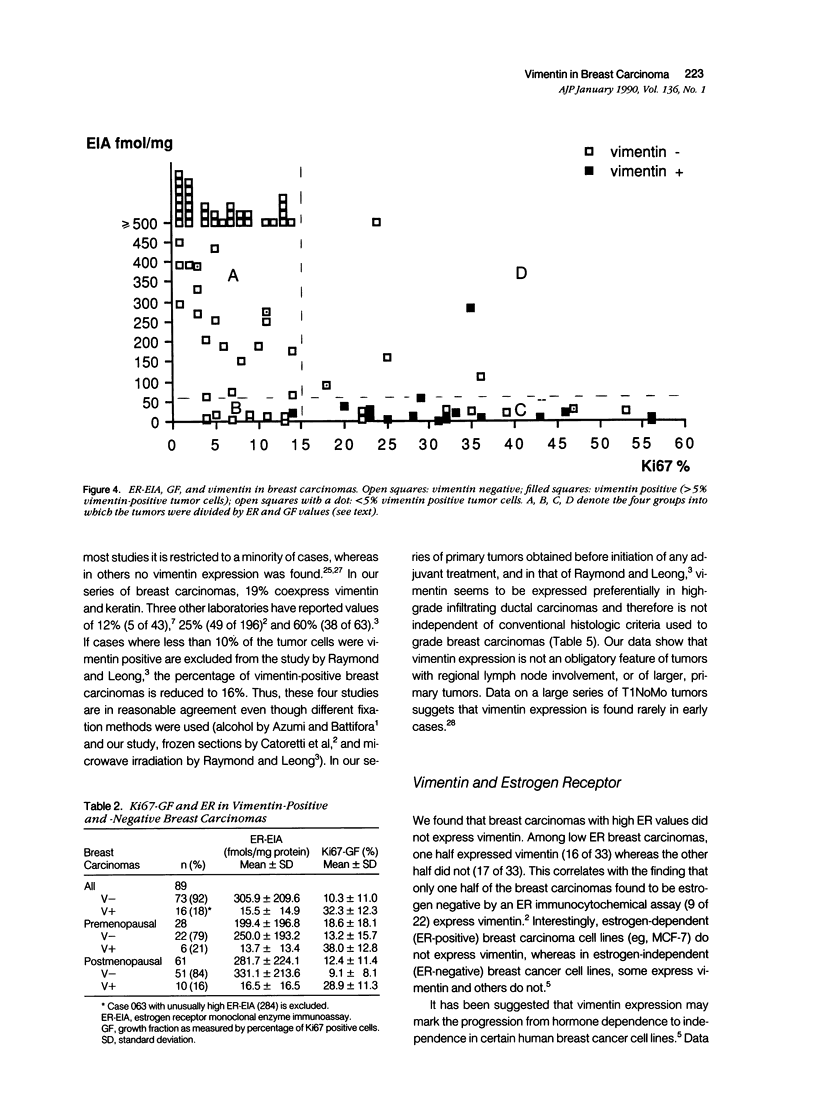



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Schauer A., Weber K. Antibodies to different intermediate filament proteins. Cell type-specific markers on paraffin-embedded human tissues. Lab Invest. 1981 Nov;45(5):427–434. [PubMed] [Google Scholar]
- Assoian R. K., Frolik C. A., Roberts A. B., Miller D. M., Sporn M. B. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984 Jan;36(1):35–41. doi: 10.1016/0092-8674(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Azumi N., Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol. 1987 Sep;88(3):286–296. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- BLOOM H. J., RICHARDSON W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957 Sep;11(3):359–377. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard N. J., Hall P. A., Lemoine N. R., Kadar N. Proliferative index in breast carcinoma determined in situ by Ki67 immunostaining and its relationship to clinical and pathological variables. J Pathol. 1987 Aug;152(4):287–295. doi: 10.1002/path.1711520407. [DOI] [PubMed] [Google Scholar]
- Barnes D. M., Fentiman I. S., Millis R. R., Rubens R. D. Who needs steroid receptor assays? Lancet. 1989 May 20;1(8647):1126–1127. doi: 10.1016/s0140-6736(89)92395-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Differential control of cytokeratins and vimentin synthesis by cell-cell contact and cell spreading in cultured epithelial cells. J Cell Biol. 1984 Oct;99(4 Pt 1):1424–1433. doi: 10.1083/jcb.99.4.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G., Andreola S., Clemente C., D'Amato L., Rilke F. Vimentin and p53 expression on epidermal growth factor receptor-positive, oestrogen receptor-negative breast carcinomas. Br J Cancer. 1988 Apr;57(4):353–357. doi: 10.1038/bjc.1988.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpin C., Andrac L., Vacheret H., Habib M. C., Devictor B., Lavaut M. N., Toga M. Multiparametric evaluation (SAMBA) of growth fraction (monoclonal Ki67) in breast carcinoma tissue sections. Cancer Res. 1988 Aug 1;48(15):4368–4374. [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Domagala W., Lasota J., Chosia M., Szadowska A., Weber K., Osborn M. Diagnosis of major tumor categories in fine-needle aspirates is more accurate when light microscopy is combined with intermediate filament typing. A study of 403 cases. Cancer. 1989 Feb 1;63(3):504–517. doi: 10.1002/1097-0142(19890201)63:3<504::aid-cncr2820630319>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Donhuijsen K., Schulz S. Prognostic significance of vimentin positivity in formalin-fixed renal cell carcinomas. Pathol Res Pract. 1989 Mar;184(3):287–291. doi: 10.1016/S0344-0338(89)80088-3. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Battini R., Kaczmarek L., Rittling S., Calabretta B., de Riel J. K., Philiponis V., Wei J. F., Baserga R. Coding sequence and growth regulation of the human vimentin gene. Mol Cell Biol. 1986 Nov;6(11):3614–3620. doi: 10.1128/mcb.6.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Pickartz H., Brotherton J., Hammerstein J., Weitzel H., Stein H. Growth fractions and estrogen receptors in human breast cancers as determined in situ with monoclonal antibodies. Am J Pathol. 1987 Dec;129(3):486–492. [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Giese G., Traub P. Induction of vimentin synthesis in a murine myeloma cell line by TPA is strongly dependent on the composition of the cell culture medium. Eur J Cell Biol. 1988 Dec;47(2):291–299. [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Gröne E., Helmchen U., Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987 Oct;129(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Guelstein V. I., Tchypysheva T. A., Ermilova V. D., Litvinova L. V., Troyanovsky S. M., Bannikov G. A. Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer. 1988 Aug 15;42(2):147–153. doi: 10.1002/ijc.2910420202. [DOI] [PubMed] [Google Scholar]
- Heuson J. C., Longeval E., Mattheiem W. H., Deboel M. C., Sylvester R. J., Leclercq G. Significance of quantitative assessment of estrogen receptors for endocrine therapy in advanced breast cancer. Cancer. 1977 May;39(5):1971–1977. doi: 10.1002/1097-0142(197705)39:5<1971::aid-cncr2820390510>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Jakesz R., Smith C. A., Aitken S., Huff K., Schuette W., Shackney S., Lippman M. Influence of cell proliferation and cell cycle phase on expression of estrogen receptor in MCF-7 breast cancer cells. Cancer Res. 1984 Feb;44(2):619–625. [PubMed] [Google Scholar]
- Kamel O. W., Franklin W. A., Ringus J. C., Meyer J. S. Thymidine labeling index and Ki-67 growth fraction in lesions of the breast. Am J Pathol. 1989 Jan;134(1):107–113. [PMC free article] [PubMed] [Google Scholar]
- Lellé R. J., Heidenreich W., Stauch G., Gerdes J. The correlation of growth fractions with histologic grading and lymph node status in human mammary carcinoma. Cancer. 1987 Jan 1;59(1):83–88. doi: 10.1002/1097-0142(19870101)59:1<83::aid-cncr2820590119>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- McGuire W. L. Current status of estrogen receptors in human breast cancer. Cancer. 1975 Aug;36(2):638–644. doi: 10.1002/1097-0142(197508)36:2+<638::aid-cncr2820360805>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- McGurrin J. F., Doria M. I., Jr, Dawson P. J., Karrison T., Stein H. O., Franklin W. A. Assessment of tumor cell kinetics by immunohistochemistry in carcinoma of breast. Cancer. 1987 May 15;59(10):1744–1750. doi: 10.1002/1097-0142(19870515)59:10<1744::aid-cncr2820591012>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Medeiros L. J., Michie S. A., Johnson D. E., Warnke R. A., Weiss L. M. An immunoperoxidase study of renal cell carcinomas: correlation with nuclear grade, cell type, and histologic pattern. Hum Pathol. 1988 Aug;19(8):980–987. doi: 10.1016/s0046-8177(88)80016-9. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Lee J. Y. Relationships of S-phase fraction of breast carcinoma in relapse to duration of remission, estrogen receptor content, therapeutic responsiveness, and duration of survival. Cancer Res. 1980 Jun;40(6):1890–1896. [PubMed] [Google Scholar]
- Meyer J. S., Prey M. U., Babcock D. S., McDivitt R. W. Breast carcinoma cell kinetics, morphology, stage, and host characteristics. A thymidine labeling study. Lab Invest. 1986 Jan;54(1):41–51. [PubMed] [Google Scholar]
- Osborne C. K., Yochmowitz M. G., Knight W. A., 3rd, McGuire W. L. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980 Dec 15;46(12 Suppl):2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Raymond W. A., Leong A. S. Co-expression of cytokeratin and vimentin intermediate filament proteins in benign and neoplastic breast epithelium. J Pathol. 1989 Apr;157(4):299–306. doi: 10.1002/path.1711570406. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Schmid E., Schiller D. L., Grund C., Stadler J., Franke W. W. Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J Cell Biol. 1983 Jan;96(1):37–50. doi: 10.1083/jcb.96.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstrini R., Daidone M. G., Di Fronzo G. Relationship between proliferative activity and estrogen receptors in breast cancer. Cancer. 1979 Aug;44(2):665–670. doi: 10.1002/1097-0142(197908)44:2<665::aid-cncr2820440237>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Silvestrini R., Daidone M. G., Gasparini G. Cell kinetics as a prognostic marker in node-negative breast cancer. Cancer. 1985 Oct 15;56(8):1982–1987. doi: 10.1002/1097-0142(19851015)56:8<1982::aid-cncr2820560816>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Striem B. J., Pace U., Zehavi U., Naim M., Lancet D. Sweet tastants stimulate adenylate cyclase coupled to GTP-binding protein in rat tongue membranes. Biochem J. 1989 May 15;260(1):121–126. doi: 10.1042/bj2600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe S. M. Monoclonal antibody technique for detection of estrogen receptors in human breast cancer: greater sensitivity and more accurate classification of receptor status than the dextran-coated charcoal method. Cancer Res. 1987 Dec 15;47(24 Pt 1):6572–6575. [PubMed] [Google Scholar]
- Thorpe S. M., Rose C., Rasmussen B. B., Mouridsen H. T., Bayer T., Keiding N. Prognostic value of steroid hormone receptors: multivariate analysis of systemically untreated patients with node negative primary breast cancer. Cancer Res. 1987 Nov 15;47(22):6126–6133. [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H. J., Schlingemann R. O., Boehmer A. L., van Driel R., Brakenhoff G. J., Ramaekers F. C. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. I. Intracellular localization during interphase. J Cell Sci. 1989 Jan;92(Pt 1):123–130. doi: 10.1242/jcs.92.1.123. [DOI] [PubMed] [Google Scholar]
- van Dierendonck J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Nuclear distribution of the Ki-67 antigen during the cell cycle: comparison with growth fraction in human breast cancer cells. Cancer Res. 1989 Jun 1;49(11):2999–3006. [PubMed] [Google Scholar]