Abstract
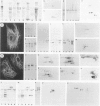
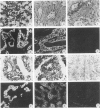
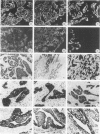
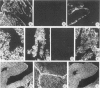
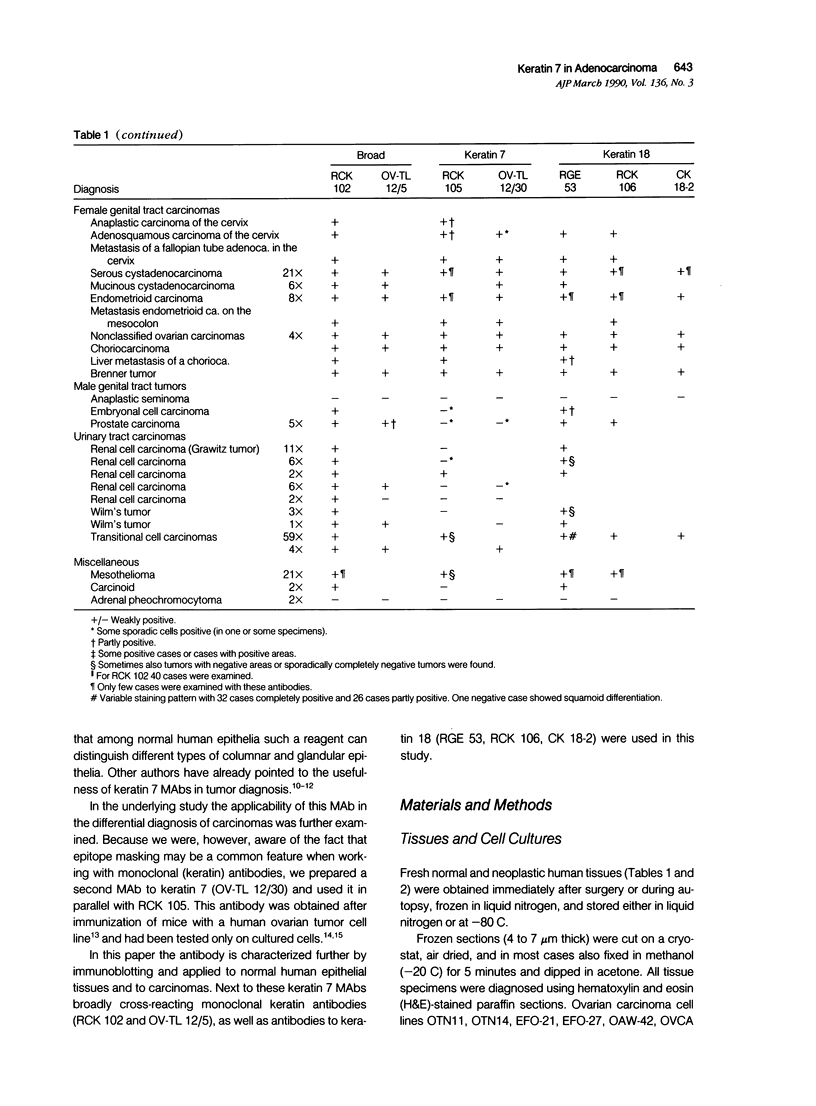
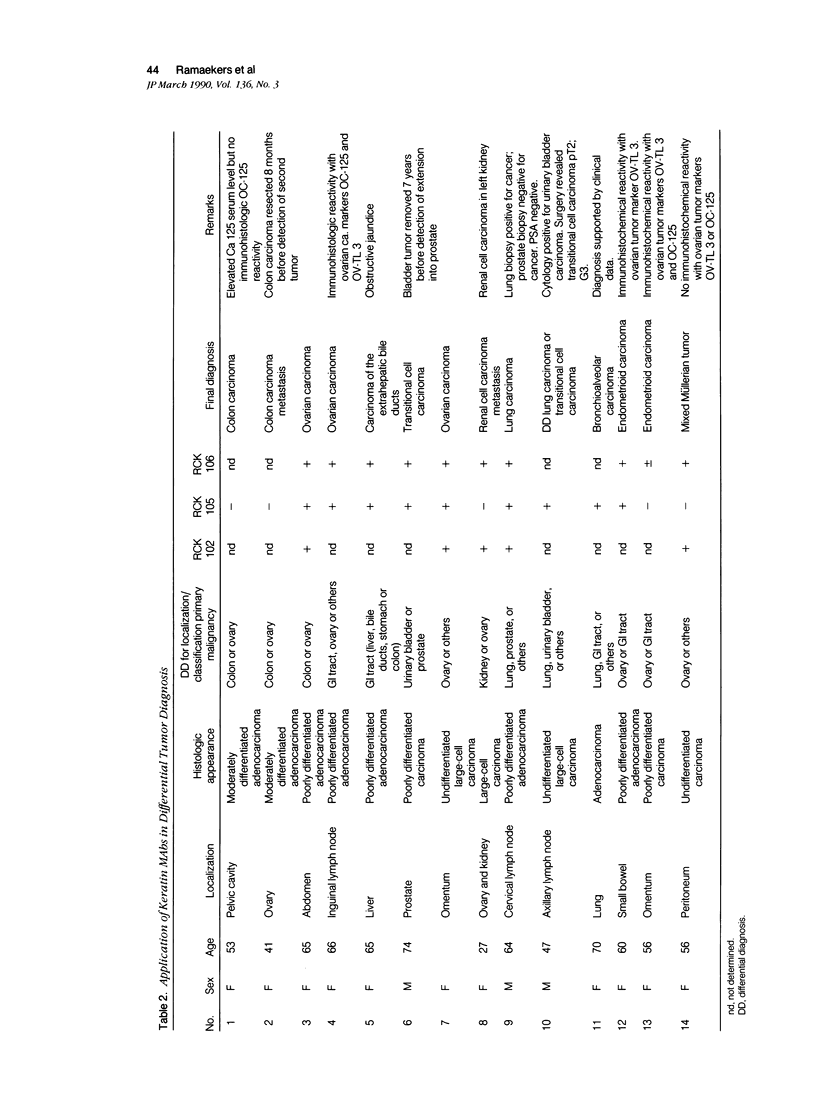
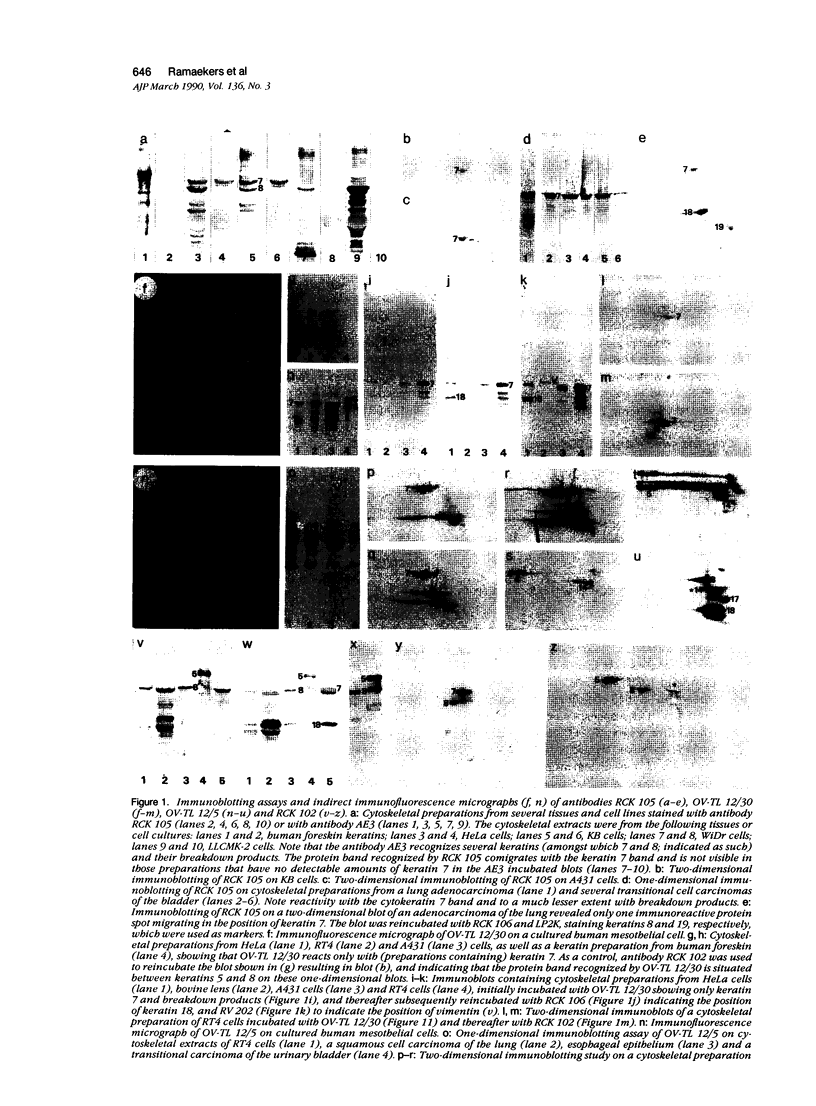
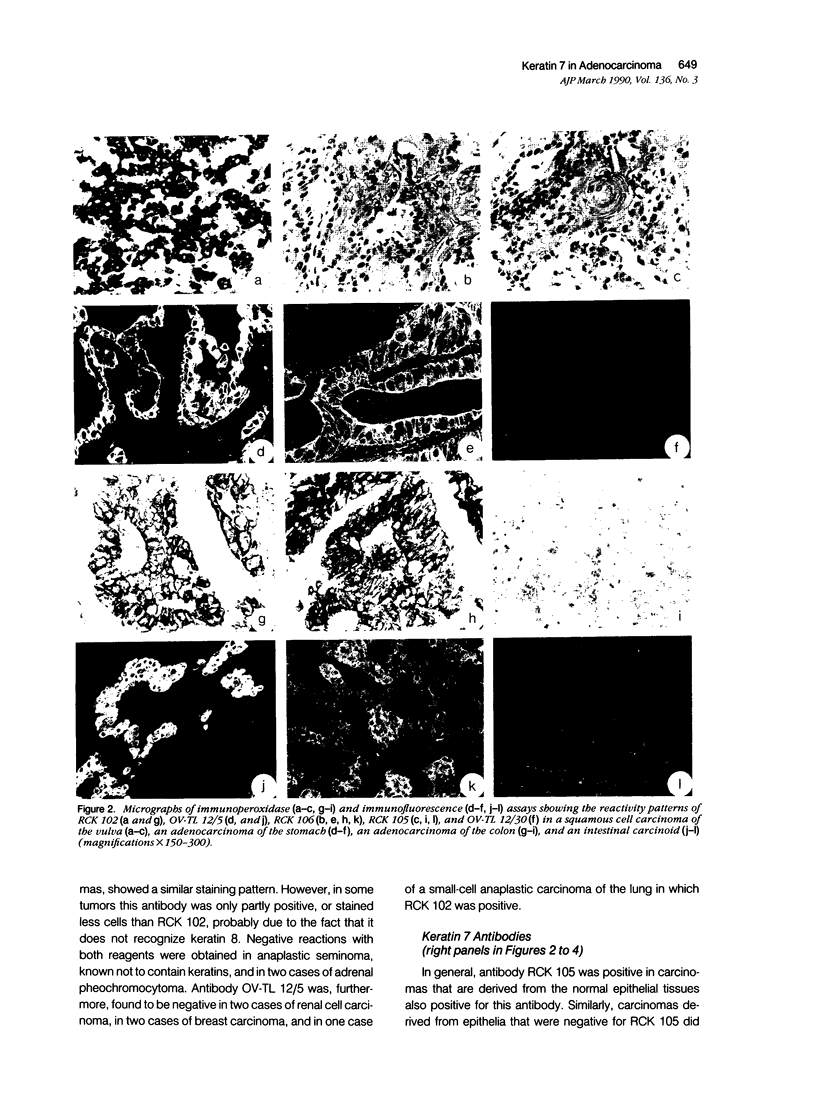
Monoclonal antibodies (MAbs) to specific keratin subtypes were prepared and characterized by immunoblotting and immunohistochemical assays on human cell cultures and normal and malignant human tissues. Chain-specific MAbs to keratin 7 (RCK 105, OV-TL 12/30) and keratin 18 (RGE 53, RCK 106, CK18-2), as well as broadly cross-reacting keratin MAbs (RCK 102, OV-TL 12/5) could be shown to react with different types of human epithelial tissues and were therefore tested for their usefulness in the differential diagnosis of carcinomas. The two broad-spectrum antibodies stained virtually all of the more than 350 carcinomas tested, especially when combined, and distinguished them from most nonepithelial tumors. The keratin 18 MAbs distinguished adenocarcinomas (which are keratin 18 positive) from most squamous cell carcinomas (which are generally keratin 18 negative). The MAbs to keratin 7 could be shown to recognize specific subtypes of adenocarcinoma and could, for example, distinguish between ovarian carcinomas (keratin 7 positive) and carcinomas of the gastrointestinal tract (keratin 7 negative), or between transitional cell carcinomas (keratin 7 positive) and prostate cancer (keratin 7 negative). In general, malignancies showed the expected keratin reactivity pattern as concluded from the keratin pattern of its cell of origin or its type of differentiation. The use of an extended series of malignancies did, however, also illustrate that exceptions to this rule exist. For example, certain antibodies to keratin 18 stained tumor areas in squamous cell carcinomas of the lung. Also a certain percentage of tumors, which generally showed no keratin 7 expression, were positive with RCK 105 or OV-TL 12/30. On the other hand, a certain percentage of tumors, which were generally positive for keratin 7, did not show a staining reaction with these MAbs. Furthermore subtle differences between reactivity patterns of different MAbs recognizing the same keratin protein were observed, both in the normal and malignant human tissues, indicating that specific keratin epitopes may be masked in certain tissues and that unmasking of such epitopes can occur with malignant progression. This phenomenon may be of some use in a further subtyping of carcinomas, especially those of the gastrointestinal tract. Despite these exceptional staining patterns, the keratin MAbs described above have proved to be useful tools in the characterization of epithelial tumors in routine histopathology and cytopathology, in which they add to a more refined diagnosis of (adeno)carcinomas.
Full text
PDF




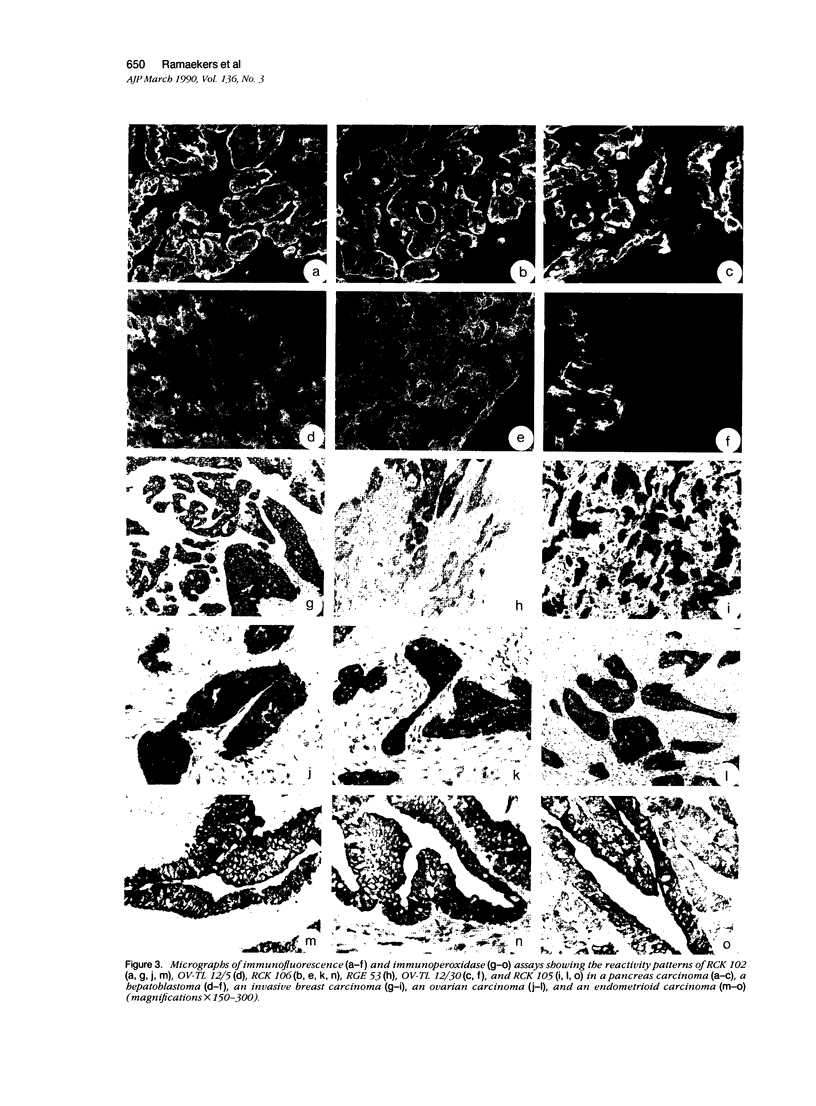





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broers J. L., Carney D. N., Klein Rot M., Schaart G., Lane E. B., Vooijs G. P., Ramaekers F. C. Intermediate filament proteins in classic and variant types of small cell lung carcinoma cell lines: a biochemical and immunochemical analysis using a panel of monoclonal and polyclonal antibodies. J Cell Sci. 1986 Jul;83:37–60. doi: 10.1242/jcs.83.1.37. [DOI] [PubMed] [Google Scholar]
- Broers J. L., Ramaekers F. C., Rot M. K., Oostendorp T., Huysmans A., van Muijen G. N., Wagenaar S. S., Vooijs G. P. Cytokeratins in different types of human lung cancer as monitored by chain-specific monoclonal antibodies. Cancer Res. 1988 Jun 1;48(11):3221–3229. [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Fischer H. P., Altmannsberger M., Weber K., Osborn M. Keratin polypeptides in malignant epithelial liver tumors. Differential diagnostic and histogenetic aspects. Am J Pathol. 1987 Jun;127(3):530–537. [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Schmid E. Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur J Cell Biol. 1979 Aug;19(3):269–275. [PubMed] [Google Scholar]
- Guelstein V. I., Tchypysheva T. A., Ermilova V. D., Litvinova L. V., Troyanovsky S. M., Bannikov G. A. Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer. 1988 Aug 15;42(2):147–153. doi: 10.1002/ijc.2910420202. [DOI] [PubMed] [Google Scholar]
- Habets J. M., Tank B., Vuzevski V. D., Brevé J., Stolz E., van Joost T. Absence of cytokeratin 8 and inconsistent expression of cytokeratins 7 and 19 in human basal cell carcinoma. Anticancer Res. 1988 Jul-Aug;8(4):611–616. [PubMed] [Google Scholar]
- Heid H. W., Moll I., Franke W. W. Patterns of expression of trichocytic and epithelial cytokeratins in mammalian tissues. I. Human and bovine hair follicles. Differentiation. 1988;37(2):137–157. doi: 10.1111/j.1432-0436.1988.tb00805.x. [DOI] [PubMed] [Google Scholar]
- James J., Lygidakis N. J., van Eyken P., Tanka A. K., Bosch K. S., Ramaekers F. C., Desmer V. Application of keratin immunocytochemistry and sirius red staining in evaluating intrahepatic changes with acute extrahepatic cholestasis due to hepatic duct carcinoma. Hepatogastroenterology. 1989 Jun;36(3):151–155. [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Moll R., Achtstätter T., Becht E., Balcarova-Ständer J., Ittensohn M., Franke W. W. Cytokeratins in normal and malignant transitional epithelium. Maintenance of expression of urothelial differentiation features in transitional cell carcinomas and bladder carcinoma cell culture lines. Am J Pathol. 1988 Jul;132(1):123–144. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Levy R., Czernobilsky B., Hohlweg-Majert P., Dallenbach-Hellweg G., Franke W. W. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest. 1983 Nov;49(5):599–610. [PubMed] [Google Scholar]
- Nagle R. B., McDaniel K. M., Clark V. A., Payne C. M. The use of antikeratin antibodies in the diagnosis of human neoplasms. Am J Clin Pathol. 1983 Apr;79(4):458–466. doi: 10.1093/ajcp/79.4.458. [DOI] [PubMed] [Google Scholar]
- Osborn M., Mazzoleni G., Santini D., Marrano D., Martinelli G., Weber K. Villin, intestinal brush border hydrolases and keratin polypeptides in intestinal metaplasia and gastric cancer; an immunohistologic study emphasizing the different degrees of intestinal and gastric differentiation in signet ring cell carcinomas. Virchows Arch A Pathol Anat Histopathol. 1988;413(4):303–312. doi: 10.1007/BF00783022. [DOI] [PubMed] [Google Scholar]
- Osborn M., van Lessen G., Weber K., Klöppel G., Altmannsberger M. Differential diagnosis of gastrointestinal carcinomas by using monoclonal antibodies specific for individual keratin polypeptides. Lab Invest. 1986 Oct;55(4):497–504. [PubMed] [Google Scholar]
- Poels L. G., Jap P. H., Ramaekers F. F., Scheres J. M., Thomas C. M., Vooijs P. G., Croes H. J., Mungyer G. Characterization of a hormone-producing ovarian carcinoma cell line. Gynecol Oncol. 1989 Feb;32(2):203–214. doi: 10.1016/s0090-8258(89)80034-4. [DOI] [PubMed] [Google Scholar]
- Poels L. G., Peters D., van Megen Y., Vooijs G. P., Verheyen R. N., Willemen A., van Niekerk C. C., Jap P. H., Mungyer G., Kenemans P. Monoclonal antibody against human ovarian tumor-associated antigens. J Natl Cancer Inst. 1986 May;76(5):781–791. [PubMed] [Google Scholar]
- Quinlan R. A., Schiller D. L., Hatzfeld M., Achtstätter T., Moll R., Jorcano J. L., Magin T. M., Franke W. W. Patterns of expression and organization of cytokeratin intermediate filaments. Ann N Y Acad Sci. 1985;455:282–306. doi: 10.1111/j.1749-6632.1985.tb50418.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Pruszczynski M., Smedts F. Cytokeratins in smooth muscle cells and smooth muscle tumours. Histopathology. 1988 May;12(5):558–561. doi: 10.1111/j.1365-2559.1988.tb01979.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Kant A., Jap P., Herman C., Vooijs P. Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumors. Use in surgical pathology. Lab Invest. 1983 Sep;49(3):353–361. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Schaart G., Herman C., Vooijs P. Cytokeratin expression during neoplastic progression of human transitional cell carcinomas as detected by a monoclonal and a polyclonal antibody. Lab Invest. 1985 Jan;52(1):31–38. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Puts J., Moesker O., Kant A., Jap P., Vooijs P. Demonstration of keratin in human adenocarcinomas. Am J Pathol. 1983 May;111(2):213–223. [PMC free article] [PubMed] [Google Scholar]
- Schaafsma H. E., Ramaekers F. C., van Muijen G. N., Lane E. B., Leigh I. M., Robben H., Huijsmans A., Ooms E. C., Ruiter D. J. Distribution of cytokeratin polypeptides in human transitional cell carcinomas, with special emphasis on changing expression patterns during tumor progression. Am J Pathol. 1990 Feb;136(2):329–343. [PMC free article] [PubMed] [Google Scholar]
- Schaafsma H. E., Ramaekers F. C., van Muijen G. N., Ooms E. C., Ruiter D. J. Distribution of cytokeratin polypeptides in epithelia of the adult human urinary tract. Histochemistry. 1989;91(2):151–159. doi: 10.1007/BF00492389. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Paterson A., Callea F., Kew M. C., Desmet V. J. Cytokeratin expression in hepatocellular carcinoma: an immunohistochemical study. Hum Pathol. 1988 May;19(5):562–568. doi: 10.1016/s0046-8177(88)80205-3. [DOI] [PubMed] [Google Scholar]
- van Eyken P., Sciot R., van Damme B., de Wolf-Peeters C., Desmet V. J. Keratin immunohistochemistry in normal human liver. Cytokeratin pattern of hepatocytes, bile ducts and acinar gradient. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):63–72. doi: 10.1007/BF00750732. [DOI] [PubMed] [Google Scholar]
- van Niekerk C. C., Jap P. H., Thomas C. M., Smeets D. F., Ramaekers F. C., Poels L. G. Marker profile of mesothelial cells versus ovarian carcinoma cells. Int J Cancer. 1989 Jun 15;43(6):1065–1071. doi: 10.1002/ijc.2910430619. [DOI] [PubMed] [Google Scholar]
- van Niekerk C. C., Poels L. G., Jap P. H., Smeets D. F., Thomas C. M., Ramaekers F. C., Vooijs G. P. Characterization of a human ovarian carcinoma cell line, OTN 14, derived from a mucinous cystadenocarcinoma. Int J Cancer. 1988 Jul 15;42(1):104–111. doi: 10.1002/ijc.2910420120. [DOI] [PubMed] [Google Scholar]