Abstract
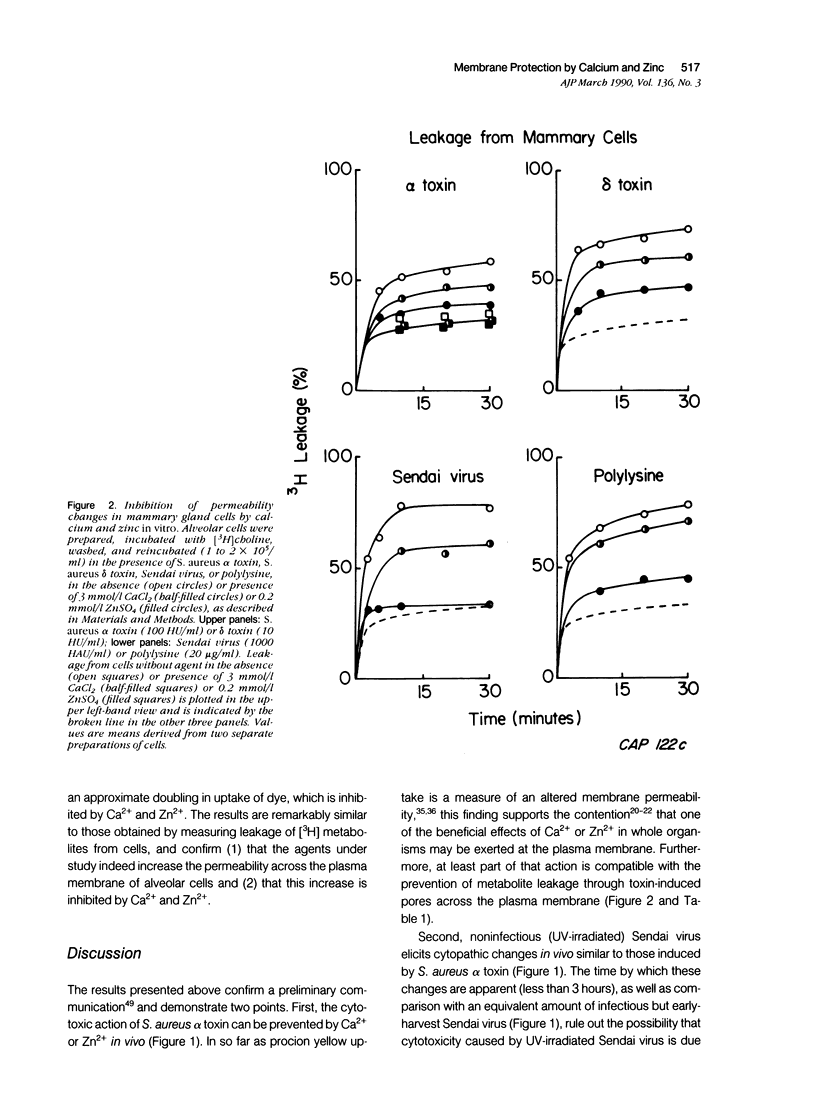
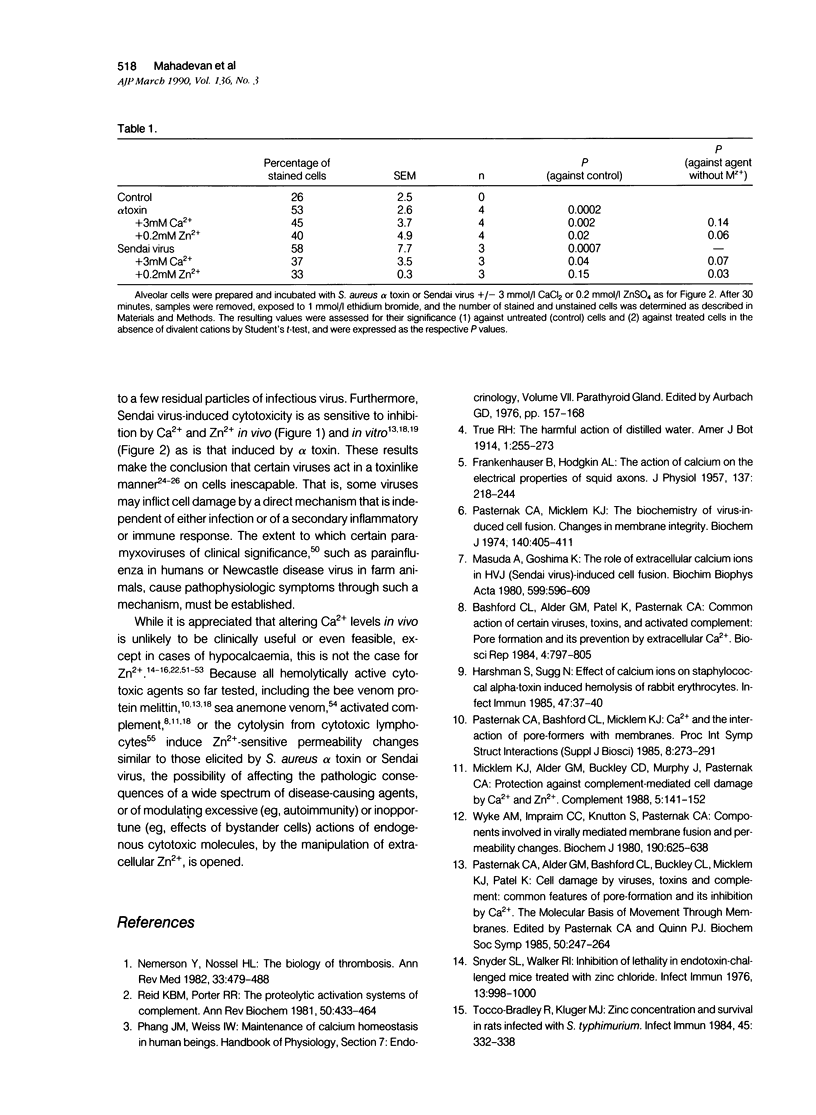
Injection of S. aureus alpha toxin or ultraviolet-inactivated Sendai virus into the mammary gland of lactating mice leads to cytopathic changes in the alveolar cells compatible with a breakdown of their permeability barrier. Simultaneous administration of Ca2+ or Zn2+ prevents such changes, as it does when added to alveolar cells in vitro. It is concluded that increased levels of Zn2+ (and under certain conditions of Ca2+) may exert a protective role that is clinically significant.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Nakib W., Higgins P. G., Barrow I., Batstone G., Tyrrell D. A. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J Antimicrob Chemother. 1987 Dec;20(6):893–901. doi: 10.1093/jac/20.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. C., Mason A. J. The effect of cell-free staphylococcal toxin on the mammary gland of the mouse. Res Vet Sci. 1974 Jan;16(1):23–26. [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Graham J. M., Menestrina G., Pasternak C. A. Ion modulation of membrane permeability: effect of cations on intact cells and on cells and phospholipid bilayers treated with pore-forming agents. J Membr Biol. 1988 Jul;103(1):79–94. doi: 10.1007/BF01871934. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Patel K., Pasternak C. A. Common action of certain viruses, toxins, and activated complement: pore formation and its prevention by extracellular Ca2+. Biosci Rep. 1984 Sep;4(9):797–805. doi: 10.1007/BF01128822. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Menestrina G., Henkart P. A., Pasternak C. A. Cell damage by cytolysin. Spontaneous recovery and reversible inhibition by divalent cations. J Immunol. 1988 Dec 1;141(11):3965–3974. [PubMed] [Google Scholar]
- Bashford C. L., Micklem K. J., Pasternak C. A. Sequential onset of permeability changes in mouse ascites cells induced by Sendai virus. Biochim Biophys Acta. 1985 Apr 11;814(2):247–255. doi: 10.1016/0005-2736(85)90442-0. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Trace element in infectious processes. Med Clin North Am. 1976 Jul;60(4):831–849. doi: 10.1016/s0025-7125(16)31864-8. [DOI] [PubMed] [Google Scholar]
- Bettger W. J., O'Dell B. L. A critical physiological role of zinc in the structure and function of biomembranes. Life Sci. 1981 Mar 30;28(13):1425–1438. doi: 10.1016/0024-3205(81)90374-x. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Serafini E. P. The permeability of normal, adenomatous, ulcerative colitic and malignant large bowel epithelial cell membranes to inulin. Br J Exp Pathol. 1985 Jun;66(3):309–315. [PMC free article] [PubMed] [Google Scholar]
- Chvapil M. Effect of zinc on cells and biomembranes. Med Clin North Am. 1976 Jul;60(4):799–812. [PubMed] [Google Scholar]
- Eby G. A., Davis D. R., Halcomb W. W. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother. 1984 Jan;25(1):20–24. doi: 10.1128/aac.25.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forda S. R., Gillies G., Kelly J. S., Micklem K. J., Pasternak C. A. Acute membrane responses to viral action. Neurosci Lett. 1982 Apr 26;29(3):237–242. doi: 10.1016/0304-3940(82)90323-8. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Baldwin J. M., Micklem K. J. Rat mast cells permeabilized with Sendai virus secrete histamine in response to Ca2+ buffered in the micromolar range. Biochem J. 1983 Mar 15;210(3):737–745. doi: 10.1042/bj2100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S., Sugg N. Effect of calcium ions on staphylococcal alpha-toxin-induced hemolysis of rabbit erythrocytes. Infect Immun. 1985 Jan;47(1):37–40. doi: 10.1128/iai.47.1.37-40.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Shimizu K., Shimizu Y. K., Ishida N. On the study of Sendai virus hemolysis. I. Complete Sendai virus lacking in hemolytic activity. Virology. 1976 May;71(1):41–47. doi: 10.1016/0042-6822(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Gomperts B. D. Rat mast cells permeabilised with streptolysin O secrete histamine in response to Ca2+ at concentrations buffered in the micromolar range. Biochim Biophys Acta. 1987 Feb 18;927(2):177–183. doi: 10.1016/0167-4889(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Impraim C. C., Foster K. A., Micklem K. J., Pasternak C. A. Nature of virally mediated changes in membrane permeability to small molecules. Biochem J. 1980 Mar 15;186(3):847–860. doi: 10.1042/bj1860847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A., Goshima K. The role of extracellular calcium ions in HVJ (Sendai virus)-induced cell fusion. Biochim Biophys Acta. 1980 Jul;599(2):596–609. doi: 10.1016/0005-2736(80)90203-5. [DOI] [PubMed] [Google Scholar]
- Menestrina G. Ionic channels formed by Staphylococcus aureus alpha-toxin: voltage-dependent inhibition by divalent and trivalent cations. J Membr Biol. 1986;90(2):177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Alder G. M., Buckley C. D., Murphy J., Pasternak C. A. Protection against complement-mediated cell damage by Ca2+ and Zn2+. Complement. 1988;5(3):141–152. doi: 10.1159/000463048. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Nyaruwe A., Pasternak C. A. Permeability changes resulting from virus-cell fusion: temperature-dependence of the contributing processes. Mol Cell Biochem. 1985 Mar;66(2):163–173. doi: 10.1007/BF00220784. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Pasternak C. A. Surface components involved in virally mediated membrane changes. Biochem J. 1977 Feb 15;162(2):405–410. doi: 10.1042/bj1620405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. P., Dankert J. R., Esser A. F. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987 Jan 1;138(1):246–253. [PubMed] [Google Scholar]
- Nemerson Y., Nossel H. L. The biology of thrombosis. Annu Rev Med. 1982;33:479–488. doi: 10.1146/annurev.me.33.020182.002403. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A. A novel form of host defence: membrane protection by Ca2+ and Zn2+. Biosci Rep. 1987 Feb;7(2):81–91. doi: 10.1007/BF01121871. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Alder G. M., Bashford C. L., Buckley C. D., Micklem K. J., Patel K. Cell damage by viruses, toxins and complement: common features of pore-formation and its inhibition by Ca2+. Biochem Soc Symp. 1985;50:247–264. [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. Permeability changes during cell fusion. J Membr Biol. 1973;14(3):293–303. doi: 10.1007/BF01868082. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. The biochemistry of virus-induced cell fusion. Changes in membrane integrity. Biochem J. 1974 Jun;140(3):405–411. doi: 10.1042/bj1400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C. A. Virus, toxin, complement: common actions and their prevention by Ca2+ or Zn2+. Bioessays. 1987 Jan;6(1):14–19. doi: 10.1002/bies.950060105. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A. Viruses as toxins. With special reference to paramyxoviruses. Brief review. Arch Virol. 1987;93(3-4):169–184. doi: 10.1007/BF01310972. [DOI] [PubMed] [Google Scholar]
- Payton B. W., Bennett M. V., Pappas G. D. Permeability and structure of junctional membranes at an electrotonic synapse. Science. 1969 Dec 26;166(3913):1641–1643. doi: 10.1126/science.166.3913.1641. [DOI] [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Koski C. L., Shin M. L., Mayer M. M. Elimination of complement channels from the plasma membranes of U937, a nucleated mammalian cell line: temperature dependence of the elimination rate. J Immunol. 1983 Sep;131(3):1411–1415. [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Snyder S. L., Walker R. I. Inhibition of lethality in endotoxin-challenged mice treated with zinc chloride. Infect Immun. 1976 Mar;13(3):998–1000. doi: 10.1128/iai.13.3.998-1000.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kravitz E. A. Neuronal geometry: determination with a technique of intracellular dye injection. Science. 1968 Oct 4;162(3849):132–134. doi: 10.1126/science.162.3849.132. [DOI] [PubMed] [Google Scholar]
- Stutchfield J., Cockcroft S. Guanine nucleotides stimulate polyphosphoinositide phosphodiesterase and exocytotic secretion from HL60 cells permeabilized with streptolysin O. Biochem J. 1988 Mar 1;250(2):375–382. doi: 10.1042/bj2500375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Möllby R. Classification of microbial, plant and animal cytolysins based on their membrane-damaging effects of human fibroblasts. Biochim Biophys Acta. 1979 Oct 19;557(1):156–169. doi: 10.1016/0005-2736(79)90098-1. [DOI] [PubMed] [Google Scholar]
- Tocco-Bradley R., Kluger M. J. Zinc concentration and survival in rats infected with Salmonella typhimurium. Infect Immun. 1984 Aug;45(2):332–338. doi: 10.1128/iai.45.2.332-338.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. M., Impraim C. C., Knutton S., Pasternak C. A. Components involved in virally mediated membrane fusion and permeability changes. Biochem J. 1980 Sep 15;190(3):625–638. doi: 10.1042/bj1900625. [DOI] [PMC free article] [PubMed] [Google Scholar]




