Abstract
Polymerase chain reaction (PCR) technology was used to examine the state of amplification of the proto-oncogene c-myc in archival ovarian carcinomas. Sequences from the c-myc gene and from a control gene were amplified simultaneously by PCR and the ratios of the two products measured. The results provided an accurate measurement of the relative number of copies of the two genes in each tumor genome if the control and test sequences amplified by PCR were of equal lengths. The results were not affected by the number of PCR cycles used. This technique should facilitate gene amplification studies in clinical medicine. Increased c-myc copy number was found in 17% of the 30 cases examined when a control from the same chromosome as c-myc was used, but in 37% of cases if a control from another chromosome was used. This underlines the importance of the genetic location of the selected control genes for such studies.
Full text
PDF



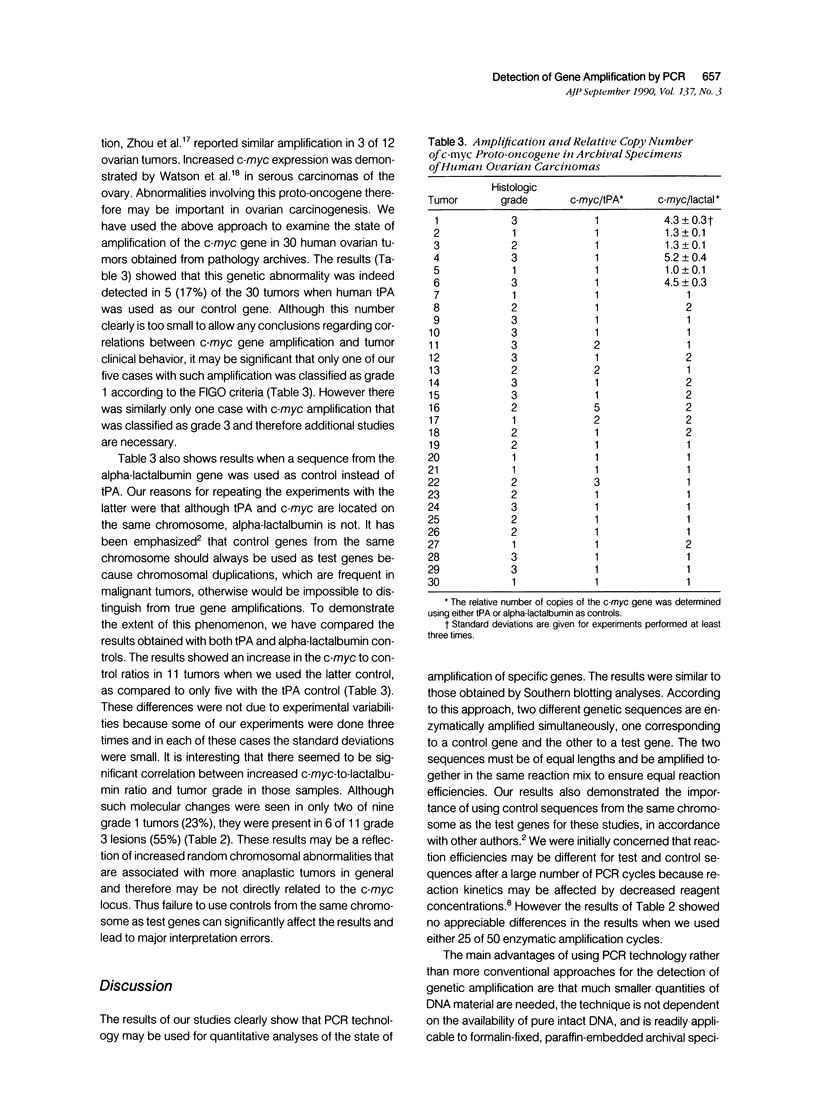

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buick R. N., Pullano R., Trent J. M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985 Aug;45(8):3668–3676. [PubMed] [Google Scholar]
- Dubeau L., Weinberg K., Jones P. A., Nichols P. W. Studies on immunoglobulin gene rearrangement in formalin-fixed, paraffin-embedded pathology specimens. Am J Pathol. 1988 Mar;130(3):588–594. [PMC free article] [PubMed] [Google Scholar]
- Frye R. A., Benz C. C., Liu E. Detection of amplified oncogenes by differential polymerase chain reaction. Oncogene. 1989 Sep;4(9):1153–1157. [PubMed] [Google Scholar]
- Gazin C., Dupont de Dinechin S., Hampe A., Masson J. M., Martin P., Stehelin D., Galibert F. Nucleotide sequence of the human c-myc locus: provocative open reading frame within the first exon. EMBO J. 1984 Feb;3(2):383–387. doi: 10.1002/j.1460-2075.1984.tb01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Roninson I., Varshavsky A., Housman D. E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):337–341. doi: 10.1073/pnas.83.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Craig R. K., Edbrooke M. R., Campbell P. N. Comparison of the nucleotide sequence of cloned human and guinea-pig pre-alpha-lactalbumin cDNA with that of chick pre-lysozyme cDNA suggests evolution from a common ancestral gene. Nucleic Acids Res. 1982 Jun 11;10(11):3503–3515. doi: 10.1093/nar/10.11.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L., Emery D. C., Davies M. S., Parker D., Craig R. K. Organization and sequence of the human alpha-lactalbumin gene. Biochem J. 1987 Mar 15;242(3):735–742. doi: 10.1042/bj2420735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ny T., Elgh F., Lund B. The structure of the human tissue-type plasminogen activator gene: correlation of intron and exon structures to functional and structural domains. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5355–5359. doi: 10.1073/pnas.81.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Roninson I. B., Chin J. E., Soffir R., Pastan I., Gottesman M. M. Multidrug resistance of DNA-mediated transformants is linked to transfer of the human mdr1 gene. Mol Cell Biol. 1986 Nov;6(11):4039–4045. doi: 10.1128/mcb.6.11.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Watson J. V., Curling O. M., Munn C. F., Hudson C. N. Oncogene expression in ovarian cancer: a pilot study of c-myc oncoprotein in serous papillary ovarian cancer. Gynecol Oncol. 1987 Oct;28(2):137–150. doi: 10.1016/0090-8258(87)90207-1. [DOI] [PubMed] [Google Scholar]
- Yasue H., Takeda A., Ishibashi M. Amplification of the c-myc gene and the elevation of its transcripts in human ovarian tumor lines. Cell Struct Funct. 1987 Feb;12(1):121–125. doi: 10.1247/csf.12.121. [DOI] [PubMed] [Google Scholar]
- Zhou D. J., Gonzalez-Cadavid N., Ahuja H., Battifora H., Moore G. E., Cline M. J. A unique pattern of proto-oncogene abnormalities in ovarian adenocarcinomas. Cancer. 1988 Oct 15;62(8):1573–1576. doi: 10.1002/1097-0142(19881015)62:8<1573::aid-cncr2820620819>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]