Abstract
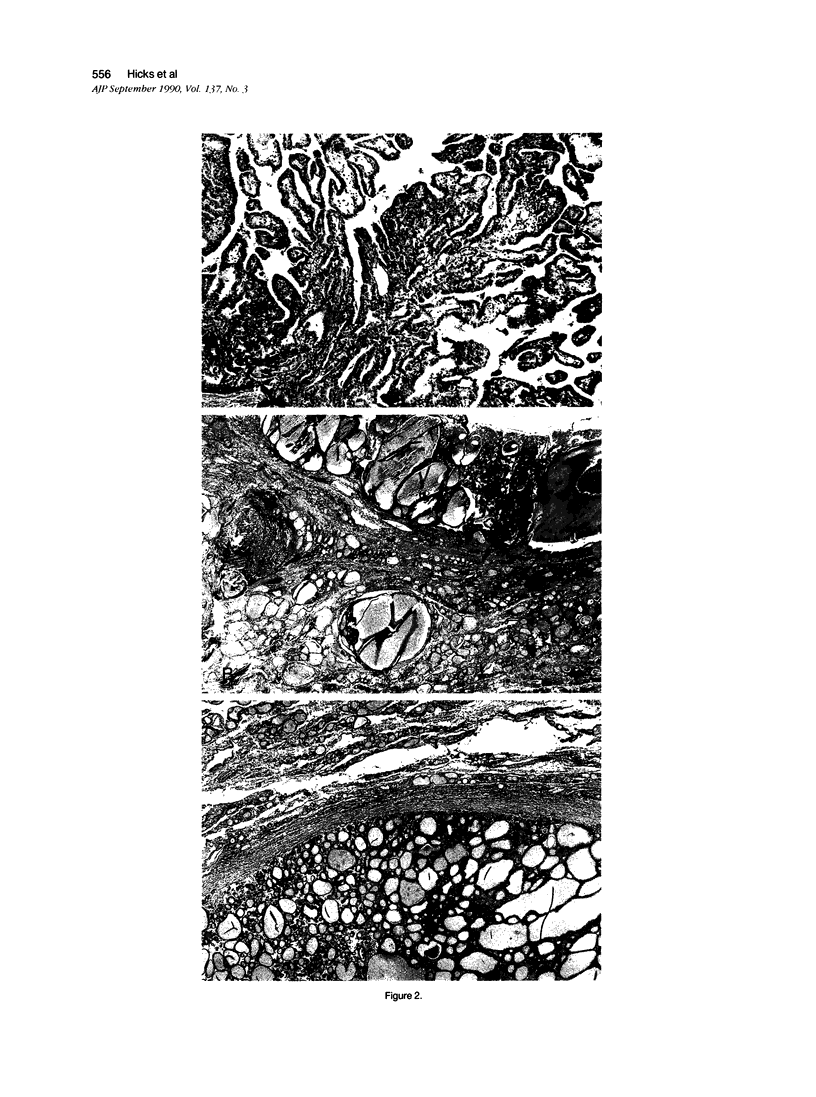
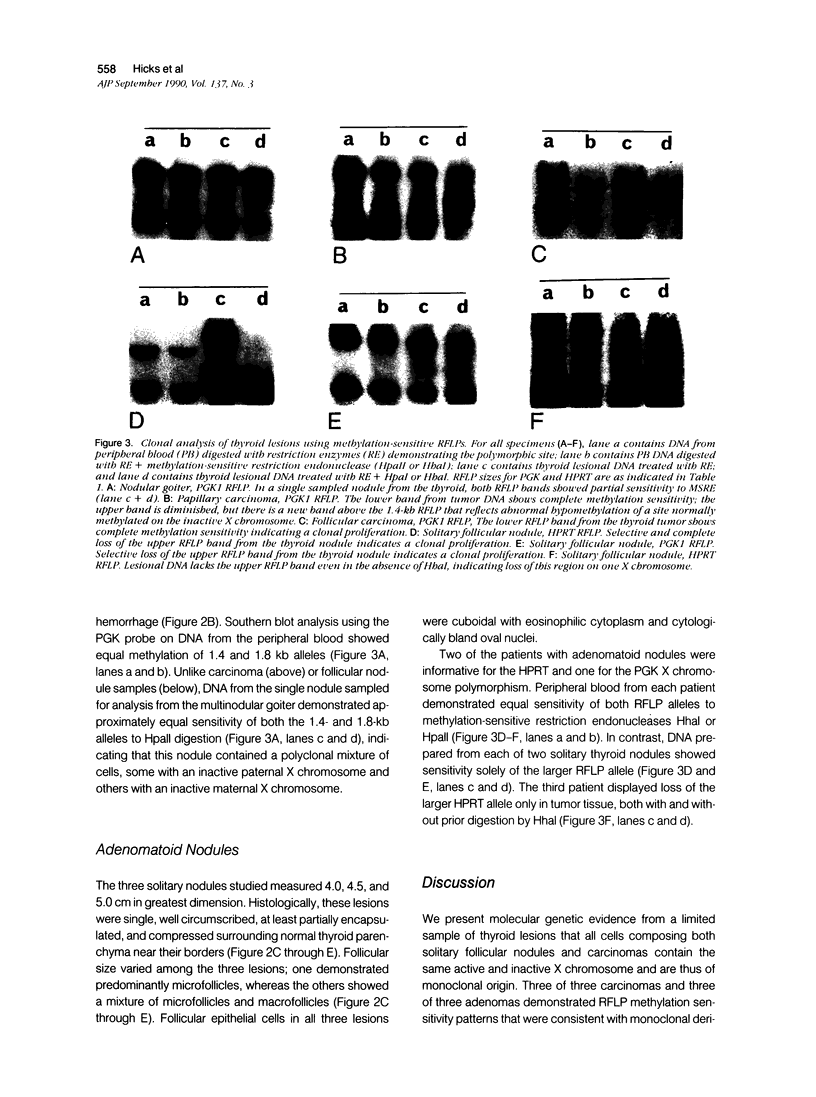
Accumulated data using functional, morphologic, and histochemical analysis suggests that follicular proliferations in the thyroid include polyclonal and monoclonal patterns with encapsulated follicular adenomas most frequently monoclonal, and other nodules generally polyclonal. However, examples of polyclonal carcinomas or adenomas raise the possibility that histologically similar lesions may arise through different pathogenetic mechanisms. The authors have performed a clonal analysis of histologically benign and malignant thyroid nodules in seven women using HPRT (hypoxanthine phosphoribosyl transferase) and PGK (phosphoglycerate kinase) restriction fragment length polymorphisms (RFLPs) on the X chromosome. These RFLPs used in concert with methylation-sensitive restriction endonucleases HpaII and HhaI permit distinction of active and inactive X chromosomes. DNA from a multinodular goiter showed equal sensitivity of both X chromosome RFLP alleles to a methylation-sensitive restriction endonuclease, consistent with a polyclonal origin. In contrast, three solitary follicular nodules and three carcinomas displayed predominant sensitivity of a single RFLP allele, consistent with a monoclonal origin. Although further detailed studies will be necessary to understand polyclonal origins reported for some adenomas, our data from a limited number of samples supports a predominantly monoclonal origin, and possible neoplastic pathogenesis, for many solitary adenomatous nodules in the thyroid.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold A., Cossman J., Bakhshi A., Jaffe E. S., Waldmann T. A., Korsmeyer S. J. Immunoglobulin-gene rearrangements as unique clonal markers in human lymphoid neoplasms. N Engl J Med. 1983 Dec 29;309(26):1593–1599. doi: 10.1056/NEJM198312293092601. [DOI] [PubMed] [Google Scholar]
- Arnold A., Kim H. G. Clonal loss of one chromosome 11 in a parathyroid adenoma. J Clin Endocrinol Metab. 1989 Sep;69(3):496–499. doi: 10.1210/jcem-69-3-496. [DOI] [PubMed] [Google Scholar]
- Arnold A., Staunton C. E., Kim H. G., Gaz R. D., Kronenberg H. M. Monoclonality and abnormal parathyroid hormone genes in parathyroid adenomas. N Engl J Med. 1988 Mar 17;318(11):658–662. doi: 10.1056/NEJM198803173181102. [DOI] [PubMed] [Google Scholar]
- Beckers C. Thyroid nodules. Clin Endocrinol Metab. 1979 Mar;8(1):181–192. doi: 10.1016/s0300-595x(79)80016-x. [DOI] [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Bird A. P. Use of restriction enzymes to study eukaryotic DNA methylation: II. The symmetry of methylated sites supports semi-conservative copying of the methylation pattern. J Mol Biol. 1978 Jan 5;118(1):49–60. doi: 10.1016/0022-2836(78)90243-7. [DOI] [PubMed] [Google Scholar]
- Bitter M. A., Le Beau M. M., Rowley J. D., Larson R. A., Golomb H. M., Vardiman J. W. Associations between morphology, karyotype, and clinical features in myeloid leukemias. Hum Pathol. 1987 Mar;18(3):211–225. doi: 10.1016/s0046-8177(87)80002-3. [DOI] [PubMed] [Google Scholar]
- Cossman J., Uppenkamp M., Sundeen J., Coupland R., Raffeld M. Molecular genetics and the diagnosis of lymphoma. Arch Pathol Lab Med. 1988 Feb;112(2):117–127. [PubMed] [Google Scholar]
- Fearon E. R., Burke P. J., Schiffer C. A., Zehnbauer B. A., Vogelstein B. Differentiation of leukemia cells to polymorphonuclear leukocytes in patients with acute nonlymphocytic leukemia. N Engl J Med. 1986 Jul 3;315(1):15–24. doi: 10.1056/NEJM198607033150103. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Hamilton S. R., Vogelstein B. Clonal analysis of human colorectal tumors. Science. 1987 Oct 9;238(4824):193–197. doi: 10.1126/science.2889267. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J. Clonal origin of human tumors. Biochim Biophys Acta. 1976 Oct 12;458(3):283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Fialkow P. J., Jackson C. E., Block M. A., Greenawald K. A. Multicellular origin of parathyroid "adenomas". N Engl J Med. 1977 Sep 29;297(13):696–698. doi: 10.1056/NEJM197709292971304. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- HULL O. H. Critical analysis of two hundred twenty-one thyroid glands; study of thyroid glands obtained at necropsy in Colorado. AMA Arch Pathol. 1955 Mar;59(3):291–311. [PubMed] [Google Scholar]
- Hsu S. H., Luk G. D., Krush A. J., Hamilton S. R., Hoover H. H., Jr Multiclonal origin of polyps in Gardner syndrome. Science. 1983 Sep 2;221(4614):951–953. doi: 10.1126/science.6879192. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M., Weinberg W. C., Berkwits L. A probabilistic model of mosaicism based on the histological analysis of chimaeric rat liver. Development. 1987 Feb;99(2):187–196. doi: 10.1242/dev.99.2.187. [DOI] [PubMed] [Google Scholar]
- Iannaccone P. M., Weinberg W. C., Deamant F. D. On the clonal origin of tumors: a review of experimental models. Int J Cancer. 1987 Jun 15;39(6):778–784. doi: 10.1002/ijc.2910390621. [DOI] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Patel P. I., Framson P. E., Caskey C. T., Chinault A. C. Fine structure of the human hypoxanthine phosphoribosyltransferase gene. Mol Cell Biol. 1986 Feb;6(2):393–403. doi: 10.1128/mcb.6.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H. J., Gerber H., Studer H., Smeds S. Pathogenesis of heterogeneity in human multinodular goiter. A study on growth and function of thyroid tissue transplanted onto nude mice. J Clin Invest. 1985 Nov;76(5):1992–2002. doi: 10.1172/JCI112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H. J., Studer H., Forster R., Gerber H. The pathogenesis of "hot" and "cold" follicles in multinodular goiters. J Clin Endocrinol Metab. 1982 Nov;55(5):941–946. doi: 10.1210/jcem-55-5-941. [DOI] [PubMed] [Google Scholar]
- Ramelli F., Studer H., Bruggisser D. Pathogenesis of thyroid nodules in multinodular goiter. Am J Pathol. 1982 Nov;109(2):215–223. [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. H., Wilkinson M. M., Ponder B. A. Detection and characterization of spatial pattern in chimaeric tissue. J Embryol Exp Morphol. 1985 Aug;88:219–230. [PubMed] [Google Scholar]
- Singer-Sam J., Keith D. H., Tani K., Simmer R. L., Shively L., Lindsay S., Yoshida A., Riggs A. D. Sequence of the promoter region of the gene for human X-linked 3-phosphoglycerate kinase. Gene. 1984 Dec;32(3):409–417. doi: 10.1016/0378-1119(84)90016-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studer H., Hunziker H. R., Ruchti C. Morphologic and functional substrate of thyrotoxicosis caused by nodular goiters. Am J Med. 1978 Aug;65(2):227–234. doi: 10.1016/0002-9343(78)90813-6. [DOI] [PubMed] [Google Scholar]
- Studer H., Peter H. J., Gerber H. Natural heterogeneity of thyroid cells: the basis for understanding thyroid function and nodular goiter growth. Endocr Rev. 1989 May;10(2):125–135. doi: 10.1210/edrv-10-2-125. [DOI] [PubMed] [Google Scholar]
- Teuscher J., Peter H. J., Gerber H., Berchtold R., Studer H. Pathogenesis of nodular goiter and its implications for surgical management. Surgery. 1988 Jan;103(1):87–93. [PubMed] [Google Scholar]
- Thomas G. A., Williams D., Williams E. D. The clonal origin of thyroid nodules and adenomas. Am J Pathol. 1989 Jan;134(1):141–147. [PMC free article] [PubMed] [Google Scholar]
- Vander J. B., Gaston E. A., Dawber T. R. The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968 Sep;69(3):537–540. doi: 10.7326/0003-4819-69-3-537. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Feinberg A. P. Use of restriction fragment length polymorphisms to determine the clonal origin of human tumors. Science. 1985 Feb 8;227(4687):642–645. doi: 10.1126/science.2982210. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- Waghorne C., Thomas M., Lagarde A., Kerbel R. S., Breitman M. L. Genetic evidence for progressive selection and overgrowth of primary tumors by metastatic cell subpopulations. Cancer Res. 1988 Nov 1;48(21):6109–6114. [PubMed] [Google Scholar]
- Waldmann T. A., Davis M. M., Bongiovanni K. F., Korsmeyer S. J. Rearrangements of genes for the antigen receptor on T cells as markers of lineage and clonality in human lymphoid neoplasms. N Engl J Med. 1985 Sep 26;313(13):776–783. doi: 10.1056/NEJM198509263131303. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]





