Abstract
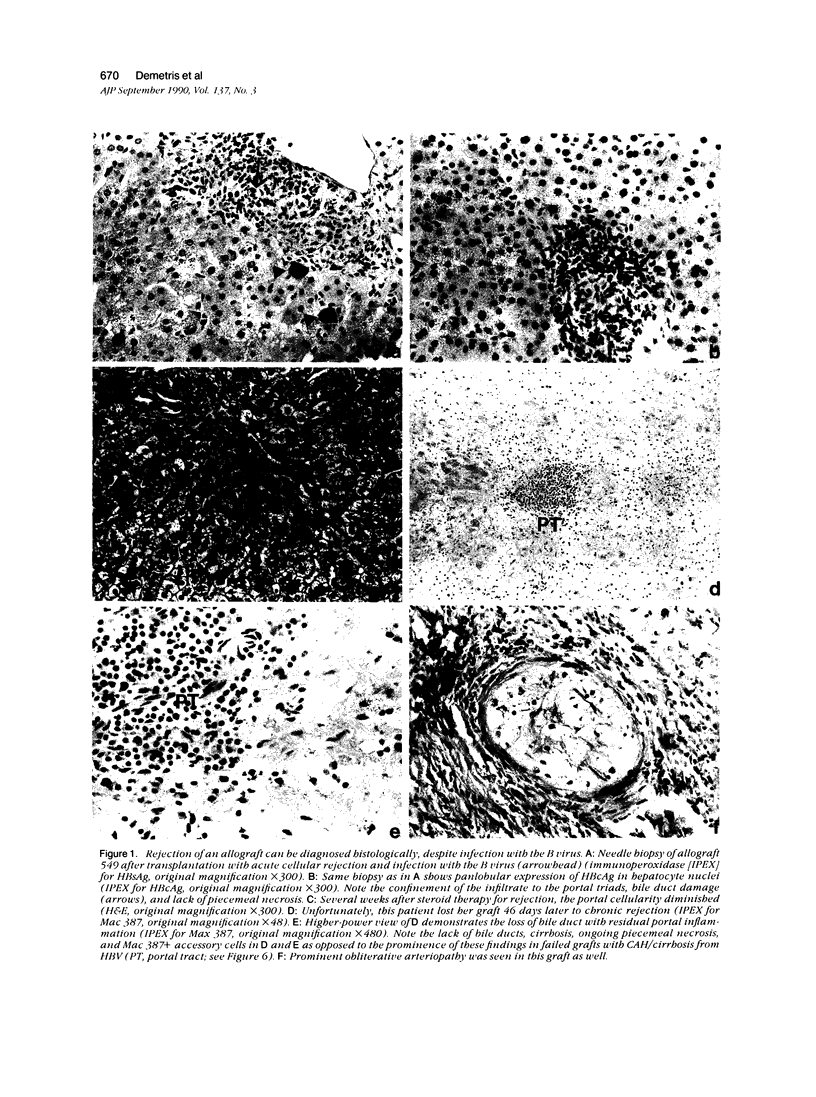
The morphologic evolution of hepatitis B virus (HBV) liver disease in 45 hepatic allograft recipients who were HBV surface-antigen positive (HBs-Ag+) at the time of liver replacement and who survived for more than 60 days was studied by routine histologic and immunocytochemical analysis of serial pathology specimens. The findings in these patients were compared to a control group of 30 individuals who were immune to the HBV (anti-HBs antibody positive), but required hepatic replacement for other reasons. Eight of the forty-five (18%) HBsAg-positive patients have no serologic evidence of HBV reinfection after transplantation. All 37 remaining patients are reinfected; 21 (47%) developed chronic active hepatitis and/or cirrhosis, 3 (7%) developed submassive necrosis, and 6 (14%) developed chronic lobular hepatitis. One patient lost her graft to chronic rejection, despite reinfection with the B virus. Four other patients (9%) developed a chronic carrier state. No long-term follow-up biopsies were available in the remaining two patients. The histologic features associated with dysfunction related to recurrent HBV infection evolved from an acute to chronic phase and were similar to hepatitis B seen in nonallografted livers. Furthermore HBV-related lesions could be separated from rejection using routine histology alone. The only exception to this conclusion was the occurrence of a peculiar HBV-related lesion in two recipients, described herein. Immunohistochemical analysis demonstrated the presence of viral antigens in almost all cases. Hepatic inflammation also was commonly present during HBV disease and consisted mostly of accessory cells and T lymphocytes. Analysis of the effect of major histocompatibility complex matching revealed no clear association between the number of class I or II matches or mismatches and the development, or pattern, of active hepatitis in the allograft. Peculiar pathologic alterations in several of the biopsies and failed allografts after HBV reinfection suggests that, under special circumstances, the B virus may be cytopathic.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chisari F. V., Ferrari C., Mondelli M. U. Hepatitis B virus structure and biology. Microb Pathog. 1989 May;6(5):311–325. doi: 10.1016/0882-4010(89)90073-9. [DOI] [PubMed] [Google Scholar]
- Chu C. M., Shyu W. C., Kuo R. W., Liaw Y. F. HLA class I antigen display on hepatocyte membrane in chronic hepatitis B virus infection: its role in the pathogenesis of chronic type B hepatitis. Hepatology. 1988 May-Jun;8(3):712–717. doi: 10.1002/hep.1840080358. [DOI] [PubMed] [Google Scholar]
- Colledan M., Gislon M., Doglia M., Fassati L. R., Ferla G., Gridelli B., Rossi G., Galmarini D. Liver transplantation in patients with B viral hepatitis and delta infection. Transplant Proc. 1987 Oct;19(5):4073–4076. [PubMed] [Google Scholar]
- Corman J. L., Putnam C. W., Iwatsuki S., Redeker A. G., Porter K. A., Peters R. L., Schröter G., Starzl T. E. Liver allograft. Its use in chronic active hepatitis with macronodular cirrhosis, hepatitis B surface antigen. Arch Surg. 1979 Jan;114(1):75–78. doi: 10.1001/archsurg.1979.01370250077016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetris A. J., Jaffe R., Sheahan D. G., Burnham J., Spero J., Iwatsuki S., Van Theil D. H., Starzl T. E. Recurrent hepatitis B in liver allograft recipients. Differentiation between viral hepatitis B and rejection. Am J Pathol. 1986 Oct;125(1):161–172. [PMC free article] [PubMed] [Google Scholar]
- Feduska N. J., Melzer J., Amend W. J., Vincenti F., Tomlanovich S., Salvatierra O., Jr Clinical management of immunosuppressive therapy for cyclosporine-treated recipients of cadaver kidney transplants at one to six months. Transplant Proc. 1986 Apr;18(2 Suppl 1):136–140. [PubMed] [Google Scholar]
- Ferrari C., Penna A., DegliAntoni A., Fiaccadori F. Cellular immune response to hepatitis B virus antigens. An overview. J Hepatol. 1988 Aug;7(1):21–33. doi: 10.1016/s0168-8278(88)80503-8. [DOI] [PubMed] [Google Scholar]
- Gerber M. A., Thung S. N. Molecular and cellular pathology of hepatitis B. Lab Invest. 1985 Jun;52(6):572–590. [PubMed] [Google Scholar]
- Johnson P. J., Wansbrough-Jones M. H., Portmann B., Eddleston A. L., Williams R., Maycook W. D., Calne R. Y. Familial HBsAg-positive hepatoma: treatment with orthotopic liver transplantation and specific immunoglobulin. Br Med J. 1978 Jan 28;1(6107):216–216. doi: 10.1136/bmj.1.6107.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauchart W., Müller R., Pichlmayr R. Long-term immunoprophylaxis of hepatitis B virus reinfection in recipients of human liver allografts. Transplant Proc. 1987 Oct;19(5):4051–4053. [PubMed] [Google Scholar]
- Pichlmayr R., Ringe B., Lauchart W., Wonigeit K. Liver transplantation. Transplant Proc. 1987 Feb;19(1 Pt 1):103–112. [PubMed] [Google Scholar]
- Rizzetto M., Macagno S., Chiaberge E., Verme G., Negro F., Marinucci G., di Giacomo C., Alfani D., Cortesini R., Milazzo F. Liver transplantation in hepatitis delta virus disease. Lancet. 1987 Aug 29;2(8557):469–471. doi: 10.1016/s0140-6736(87)91789-2. [DOI] [PubMed] [Google Scholar]