Abstract
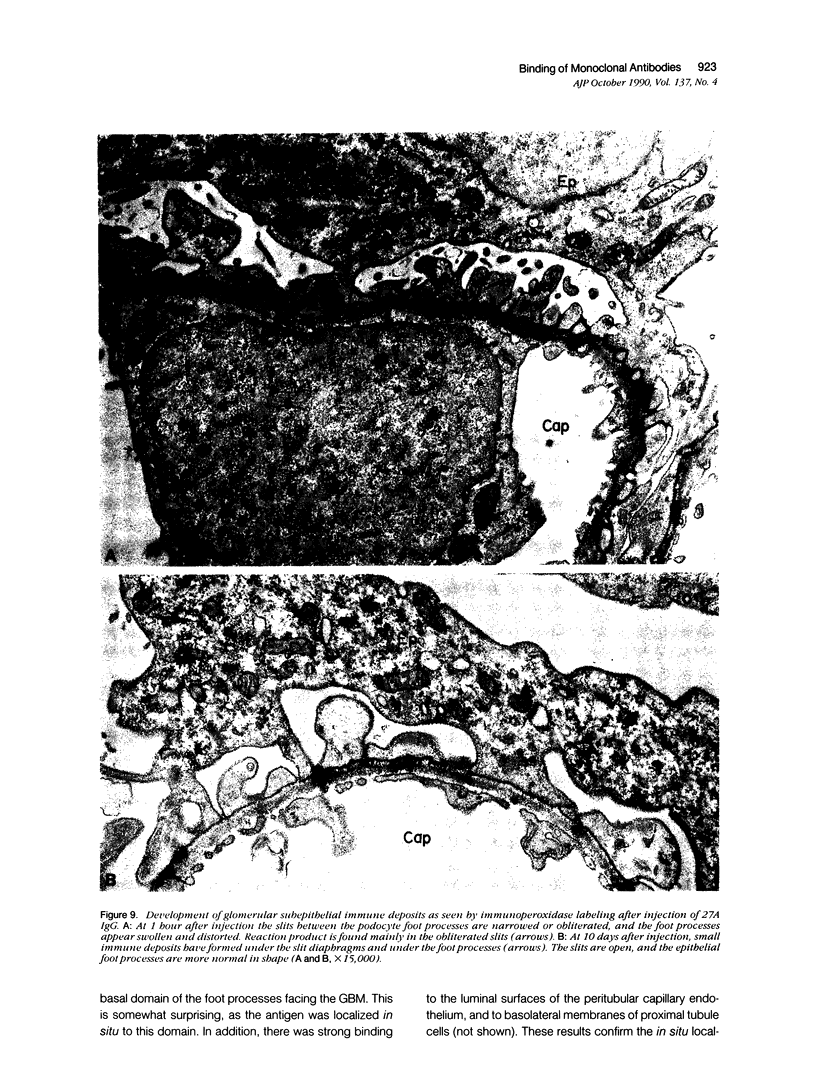
The antigens recognized by seven monoclonal antibodies (MAbs) raised against rat glomerular proteins were localized, and the sites of binding of the MAbs after in vivo injection were determined by immunoelectron microscopy. The antigens were localized in situ by immunoperoxidase and immunogold labeling to different domains and microdomains of the glomerular endothelium and epithelium. 23A recognized an antigen expressed exclusively on the luminal (apical) domain of the endothelium. 5A (anti-podocalyxin) and 26C (anti-DPPIV) recognized antigens expressed on the apical domains of both the endothelium and podocytes. 13A, 14A, 20B (anti-gp330), and 27A recognized antigens restricted to podocytes in the glomerulus. The 13A antigen was present on their basal surface and the 27A and 14A antigens were expressed on both their apical and basal domains. The 14A antigen also was associated with the filtration slit membranes. All these MAbS bound to their antigens after injection in vivo. Those that recognize endothelial antigens were rapidly cleared from the circulation and rapidly disappeared from glomeruli, whereas those that recognize epithelial antigens persisted in the circulation and were detectable in glomeruli for hours or days. The sites of binding of the MAbs differed: 23A and 5A IgG (antipodocalyxin) bound exclusively to the luminal domain of the endothelium, whereas 26C IgG (anti-DPPIV) bound to both the luminal endothelial membrane and the apical and basal domains of podocytes. The MAbs that recognize podocyte antigens bound to different domains of the podocyte plasmalemma: 13A and 27A IgGs to the basal domain, 14A to the slit membranes, and 20B to coated pits on the entire plasma membrane. 27A IgG led to the formation of small subepithelial immune deposits that remained up to 10 days. It is concluded that 1) glomerular membrane proteins vary considerably in their distribution among plasmalemmal domains and microdomains of endothelial and epithelial cells; 2) virtually all structures in the glomerulus and all domains and micro-domains of the endothelium and podocyte are accessible to circulating antibodies; and 3) the fate of immune complexes formed by binding to glomerular components varies with the location of the antigen within the glomerulus, with those that bind to the basal domain and slit membranes of the podocyte persisting longer than the others.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegri L., Brianti E., Chatelet F., Manara G. C., Ronco P., Verroust P. Polyvalent antigen-antibody interactions are required for the formation of electron-dense immune deposits in passive Heymann's nephritis. Am J Pathol. 1986 Oct;125(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- Andres G., Brentjens J. R., Caldwell P. R., Camussi G., Matsuo S. Formation of immune deposits and disease. Lab Invest. 1986 Nov;55(5):510–520. [PubMed] [Google Scholar]
- Brown W. J., Farquhar M. G. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984 Feb;36(2):295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Sawada H., Farquhar M. G. Immunoelectron microscopy in kidney research: some contributions and limitations. Kidney Int. 1986 Aug;30(2):229–245. doi: 10.1038/ki.1986.175. [DOI] [PubMed] [Google Scholar]
- Matsuo S., Fukatsu A., Taub M. L., Caldwell P. R., Brentjens J. R., Andres G. Glomerulonephritis induced in the rabbit by antiendothelial antibodies. J Clin Invest. 1987 Jun;79(6):1798–1811. doi: 10.1172/JCI113021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Miettinen A., Dekan G., Farquhar M. G. Monoclonal antibodies against membrane proteins of the rat glomerulus. Immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol. 1990 Oct;137(4):929–944. [PMC free article] [PubMed] [Google Scholar]
- Miettinen A., Stow J. L., Mentone S., Farquhar M. G. Antibodies to basement membrane heparan sulfate proteoglycans bind to the laminae rarae of the glomerular basement membrane (GBM) and induce subepithelial GBM thickening. J Exp Med. 1986 May 1;163(5):1064–1084. doi: 10.1084/jem.163.5.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa M., Matsui K., Oite T., Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol. 1988 Aug 1;141(3):807–814. [PubMed] [Google Scholar]
- Ronco P., Allegri L., Melcion C., Pirotsky E., Appay M. D., Bariety J., Pontillon F., Verroust P. A monoclonal antibody to brush border and passive Heymann nephritis. Clin Exp Immunol. 1984 Feb;55(2):319–332. [PMC free article] [PubMed] [Google Scholar]
- Ronco P., Neale T. J., Wilson C. B., Galceran M., Verroust P. An immunopathologic study of a 330-kD protein defined by monoclonal antibodies and reactive with anti-RTE alpha 5 antibodies and kidney eluates from active Heymann nephritis. J Immunol. 1986 Jan;136(1):125–130. [PubMed] [Google Scholar]
- Sawada H., Stukenbrok H., Kerjaschki D., Farquhar M. G. Epithelial polyanion (podocalyxin) is found on the sides but not the soles of the foot processes of the glomerular epithelium. Am J Pathol. 1986 Nov;125(2):309–318. [PMC free article] [PubMed] [Google Scholar]
- Schnabel E., Dekan G., Miettinen A., Farquhar M. G. Biogenesis of podocalyxin--the major glomerular sialoglycoprotein--in the newborn rat kidney. Eur J Cell Biol. 1989 Apr;48(2):313–326. [PubMed] [Google Scholar]
- Seiler M. W., Rennke H. G., Venkatachalam M. A., Cotran R. S. Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest. 1977 Jan;36(1):48–61. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]