Abstract
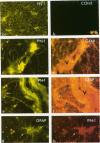
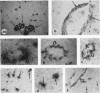
Protease nexin-1 (PN-1) is a potent thrombin inhibitor that is identical to the glia-derived neurite-promoting factor or glia-derived nexin. Here we report immunocytochemical studies of adult human cerebral cortex that revealed the presence of strong immunoreactivity for PN-1 in capillaries and in the smooth muscle cells of arteries and arterioles. Expression of PN-1 was also abundant in astroglial processes in the parenchyma and in perivascular astroglial endfeet of human cerebral cortex. In situ hybridization with an 35S-labeled RNA antisense probe for PN-1 resulted in significant labeling of astrocytes and blood vessels. Because thrombin is known to cause retraction of neurites and modification of astrocytic morphology at low concentrations, PN-1 around blood vessels may play a major protective role against extravasation of thrombin and possibly other serine protease into the human brain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh K. P., Gurwitz D., Cunningham D. D., Bradshaw R. A. Reciprocal modulation of astrocyte stellation by thrombin and protease nexin-1. J Neurochem. 1990 May;54(5):1735–1743. doi: 10.1111/j.1471-4159.1990.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Choi B. H. Developmental events during the early stages of cerebral cortical neurogenesis in man. A correlative light, electron microscopic, immunohistochemical and Golgi study. Acta Neuropathol. 1988;75(5):441–447. doi: 10.1007/BF00687130. [DOI] [PubMed] [Google Scholar]
- Choi B. H. Glial fibrillary acidic protein in radial glia of early human fetal cerebrum: a light and electron microscopic immunoperoxidase study. J Neuropathol Exp Neurol. 1986 Jul;45(4):408–418. doi: 10.1097/00005072-198607000-00003. [DOI] [PubMed] [Google Scholar]
- Choi B. H., Lapham L. W. Radial glia in the human fetal cerebrum: a combined Golgi, immunofluorescent and electron microscopic study. Brain Res. 1978 Jun 16;148(2):295–311. doi: 10.1016/0006-8993(78)90721-7. [DOI] [PubMed] [Google Scholar]
- Choi B. H., Matthias S. C. Cortical dysplasia associated with massive ectopia of neurons and glial cells within the subarachnoid space. Acta Neuropathol. 1987;73(2):105–109. doi: 10.1007/BF00693774. [DOI] [PubMed] [Google Scholar]
- Delacourte A., Defossez A., Persuy P., Peers M. C. Observation of morphological relationships between angiopathic blood vessels and degenerative neurites in Alzheimer's disease. Virchows Arch A Pathol Anat Histopathol. 1987;411(3):199–204. doi: 10.1007/BF00735024. [DOI] [PubMed] [Google Scholar]
- Eaton D. L., Baker J. B. Evidence that a variety of cultured cells secrete protease nexin and produce a distinct cytoplasmic serine protease-binding factor. J Cell Physiol. 1983 Nov;117(2):175–182. doi: 10.1002/jcp.1041170207. [DOI] [PubMed] [Google Scholar]
- Farrell D. H., Cunningham D. D. Human fibroblasts accelerate the inhibition of thrombin by protease nexin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6858–6862. doi: 10.1073/pnas.83.18.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell D. H., Wagner S. L., Yuan R. H., Cunningham D. D. Localization of protease nexin-1 on the fibroblast extracellular matrix. J Cell Physiol. 1988 Feb;134(2):179–188. doi: 10.1002/jcp.1041340203. [DOI] [PubMed] [Google Scholar]
- Gloor S., Odink K., Guenther J., Nick H., Monard D. A glia-derived neurite promoting factor with protease inhibitory activity belongs to the protease nexins. Cell. 1986 Dec 5;47(5):687–693. doi: 10.1016/0092-8674(86)90511-8. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Grabham P. W., Gallimore M. J., Gallimore P. H. Modulation of morphological differentiation of human neuroepithelial cells by serine proteases: independence from blood coagulation. EMBO J. 1989 Aug;8(8):2209–2215. doi: 10.1002/j.1460-2075.1989.tb08344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Neurite outgrowth activity of protease nexin-1 on neuroblastoma cells requires thrombin inhibition. J Cell Physiol. 1990 Jan;142(1):155–162. doi: 10.1002/jcp.1041420119. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Cunningham D. D. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci U S A. 1988 May;85(10):3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J. A., Mann D. M., Wester P., Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer's disease. Neurobiol Aging. 1986 Nov-Dec;7(6):489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Seeds N. W. Plasminogen activator secretion by granule neurons in cultures of developing cerebellum. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7810–7814. doi: 10.1073/pnas.78.12.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitt P., Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980 Oct 1;193(3):815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- Lindner J., Guenther J., Nick H., Zinser G., Antonicek H., Schachner M., Monard D. Modulation of granule cell migration by a glia-derived protein. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4568–4571. doi: 10.1073/pnas.83.12.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier R., Spreyer P., Ortmann R., Harel A., Monard D. Induction of glia-derived nexin after lesion of a peripheral nerve. Nature. 1989 Nov 30;342(6249):548–550. doi: 10.1038/342548a0. [DOI] [PubMed] [Google Scholar]
- Monard D., Niday E., Limat A., Solomon F. Inhibition of protease activity can lead to neurite extension in neuroblastoma cells. Prog Brain Res. 1983;58:359–364. doi: 10.1016/S0079-6123(08)60037-0. [DOI] [PubMed] [Google Scholar]
- Moonen G., Grau-Wagemans M. P., Selak I. Plasminogen activator-plasmin system and neuronal migration. Nature. 1982 Aug 19;298(5876):753–755. doi: 10.1038/298753a0. [DOI] [PubMed] [Google Scholar]
- Perraud F., Besnard F., Sensenbrenner M., Labourdette G. Thrombin is a potent mitogen for rat astroblasts but not for oligodendroblasts and neuroblasts in primary culture. Int J Dev Neurosci. 1987;5(3):181–188. doi: 10.1016/0736-5748(87)90028-1. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972 May;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Reinhard E., Meier R., Halfter W., Rovelli G., Monard D. Detection of glia-derived nexin in the olfactory system of the rat. Neuron. 1988 Jul;1(5):387–394. doi: 10.1016/0896-6273(88)90188-2. [DOI] [PubMed] [Google Scholar]
- Rosenblatt D. E., Cotman C. W., Nieto-Sampedro M., Rowe J. W., Knauer D. J. Identification of a protease inhibitor produced by astrocytes that is structurally and functionally homologous to human protease nexin-I. Brain Res. 1987 Jul 7;415(1):40–48. doi: 10.1016/0006-8993(87)90267-8. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Van Nostrand W. E., Wagner S. L., Cunningham D. D. Purification of a form of protease nexin 1 that binds heparin with a low affinity. Biochemistry. 1988 Mar 22;27(6):2176–2181. doi: 10.1021/bi00406a054. [DOI] [PubMed] [Google Scholar]
- Wagner S. L., Geddes J. W., Cotman C. W., Lau A. L., Gurwitz D., Isackson P. J., Cunningham D. D. Protease nexin-1, an antithrombin with neurite outgrowth activity, is reduced in Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8284–8288. doi: 10.1073/pnas.86.21.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S. L., Lau A. L., Cunningham D. D. Binding of protease nexin-1 to the fibroblast surface alters its target proteinase specificity. J Biol Chem. 1989 Jan 5;264(1):611–615. [PubMed] [Google Scholar]
- Wagner S. L., Van Nostrand W. E., Lau A. L., Cunningham D. D. Monoclonal antibodies to protease nexin 1 that differentially block its inhibition of target proteases. Biochemistry. 1988 Mar 22;27(6):2173–2176. doi: 10.1021/bi00406a053. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Kozlowski P. B. Evidence for blood-brain barrier changes in senile dementia of the Alzheimer type (SDAT). Ann N Y Acad Sci. 1982;396:119–129. doi: 10.1111/j.1749-6632.1982.tb26848.x. [DOI] [PubMed] [Google Scholar]
- Zurn A. D., Nick H., Monard D. A glia-derived nexin promotes neurite outgrowth in cultured chick sympathetic neurons. Dev Neurosci. 1988;10(1):17–24. doi: 10.1159/000111951. [DOI] [PubMed] [Google Scholar]