Abstract
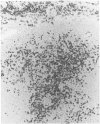
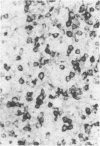
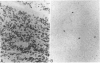
The identification of immunoglobulin protein in routinely fixed and paraffin-embedded sections using antibodies combined with immunoperoxidase or similar techniques of detection is often problematic. We developed an in situ hybridization methodology for the identification of light-chain mRNA that is applicable to formalin-fixed, paraffin-embedded tissues, using either radiolabeled or biotinylated oligonucleotide probes based on the kappa and lambda light-chain gene-constant regions. Reactive plasma cells can be consistently identified in reactive lymphoid tissues, and a monotypic pattern of light-chain mRNA restriction was seen in each of eight cases of multiple myeloma/plasmacytoma. Immunoblasts and germinal center cells also are labeled in reactive lymphoid tissues. Using 355-labeled probes, 29 of 93 cases (30%) of non-Hodgkin's lymphomas had detectable light-chain mRNA, while 19% of non-Hodgkin's lymphomas were positive using biotinylated probes.
Full text
PDF
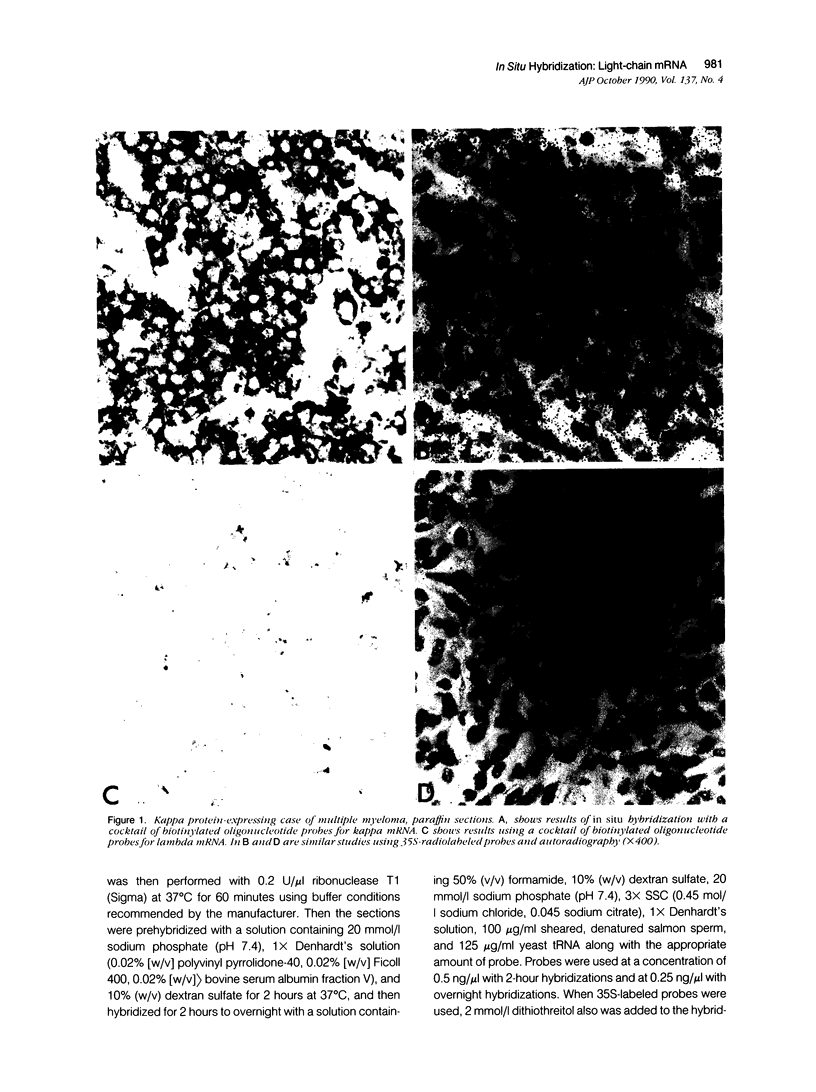
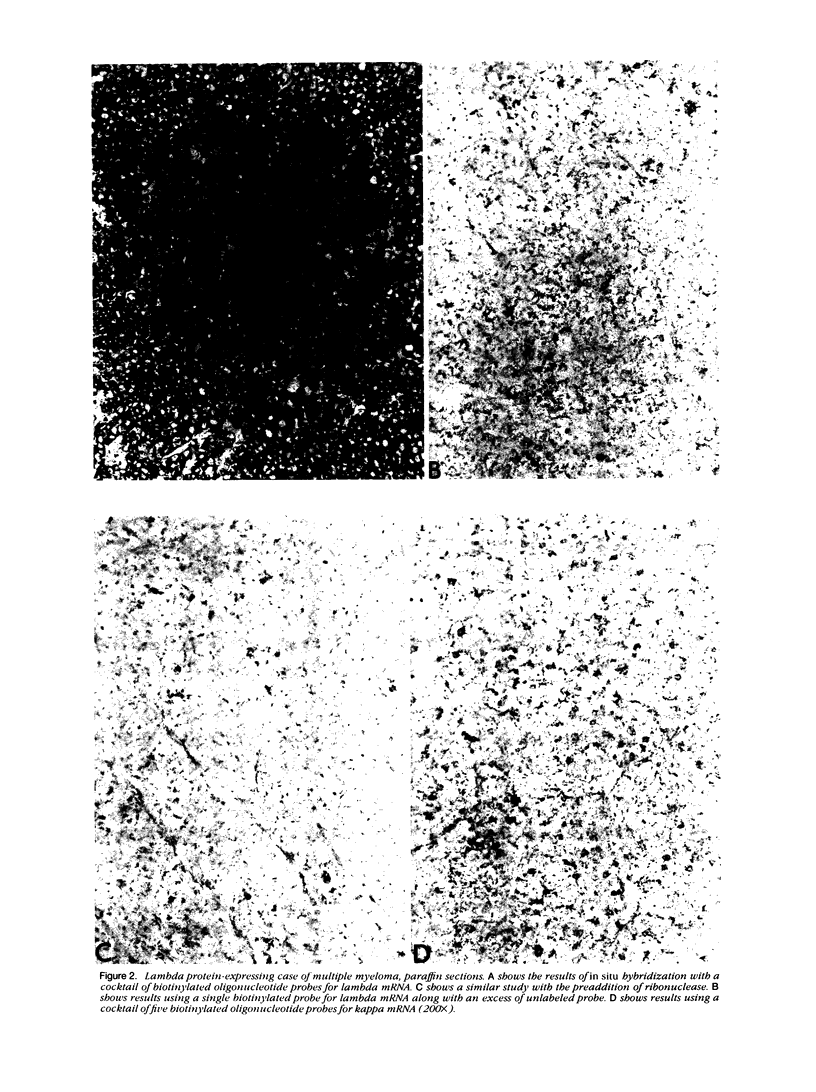

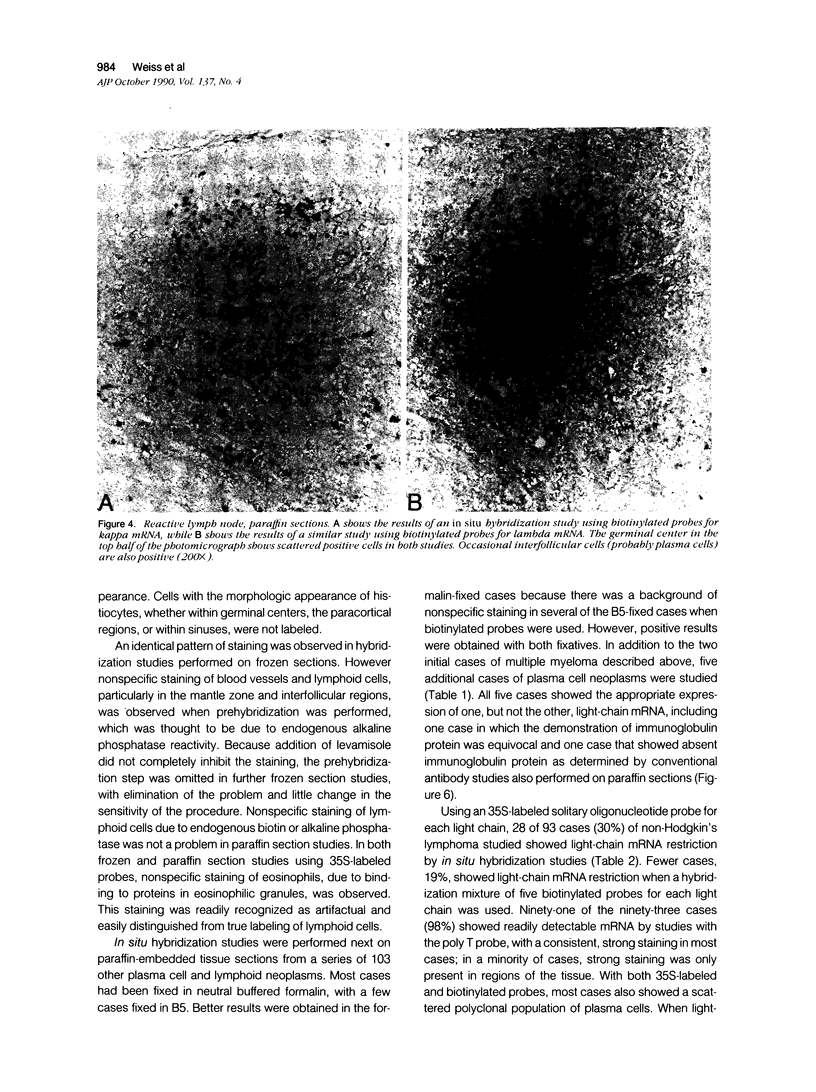

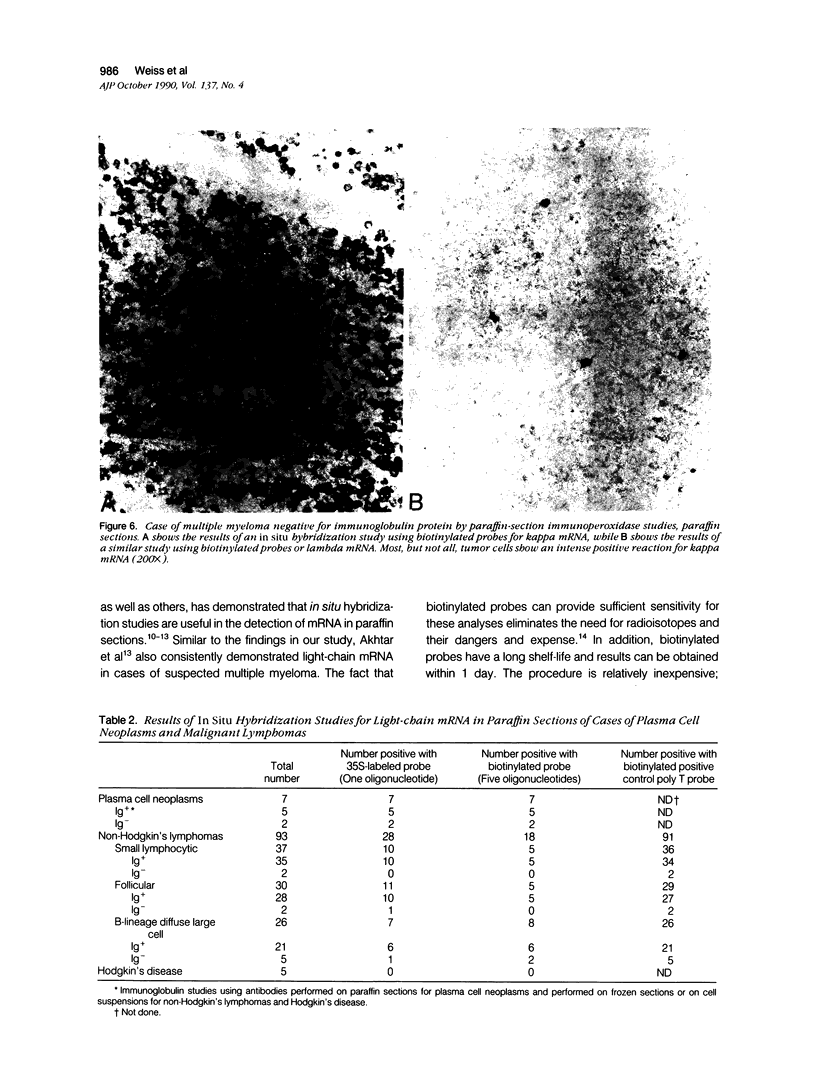
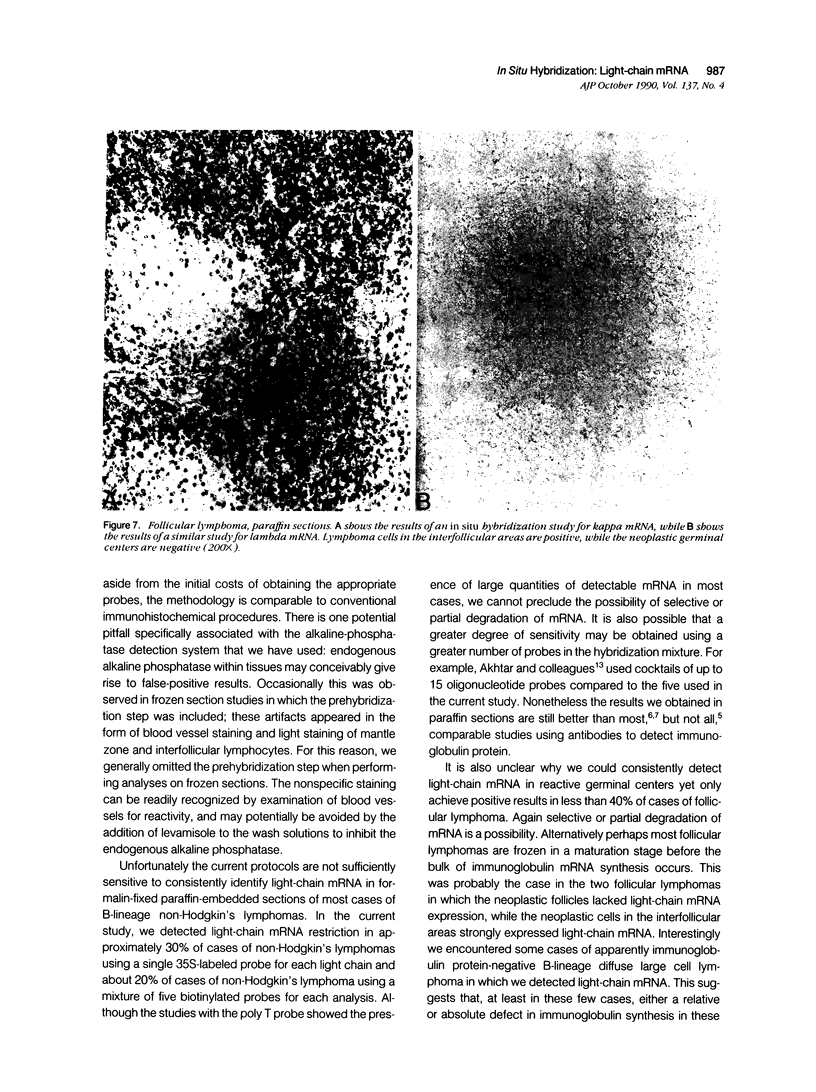

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar N., Ruprai A., Pringle J. H., Lauder I., Durrant S. T. In situ hybridization detection of light chain mRNA in routine bone marrow trephines from patients with suspected myeloma. Br J Haematol. 1989 Nov;73(3):296–301. doi: 10.1111/j.1365-2141.1989.tb07743.x. [DOI] [PubMed] [Google Scholar]
- Guitteny A. F., Fouque B., Mougin C., Teoule R., Bloch B. Histological detection of messenger RNAs with biotinylated synthetic oligonucleotide probes. J Histochem Cytochem. 1988 Jun;36(6):563–571. doi: 10.1177/36.6.3259249. [DOI] [PubMed] [Google Scholar]
- Hankin R. C., Lloyd R. V. Detection of messenger RNA in routinely processed tissue sections with biotinylated oligonucleotide probes. Am J Clin Pathol. 1989 Aug;92(2):166–171. doi: 10.1093/ajcp/92.2.166. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hollis G. F., Korsmeyer S. J., Waldmann T. A., Leder P. Clustered arrangement of immunoglobulin lambda constant region genes in man. Nature. 1981 Dec 10;294(5841):536–540. doi: 10.1038/294536a0. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Max E. E., Seidman J. G., Maizel J. V., Jr, Leder P. Cloned human and mouse kappa immunoglobulin constant and J region genes conserve homology in functional segments. Cell. 1980 Nov;22(1 Pt 1):197–207. doi: 10.1016/0092-8674(80)90168-3. [DOI] [PubMed] [Google Scholar]
- Lewis M. E., Sherman T. G., Watson S. J. In situ hybridization histochemistry with synthetic oligonucleotides: strategies and methods. Peptides. 1985;6 (Suppl 2):75–87. doi: 10.1016/0196-9781(85)90138-x. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Jin L., Fields K. Detection of chromogranins A and B in endocrine tissues with radioactive and biotinylated oligonucleotide probes. Am J Surg Pathol. 1990 Jan;14(1):35–43. doi: 10.1097/00000478-199001000-00004. [DOI] [PubMed] [Google Scholar]
- Norton A. J., Isaacson P. G. Detailed phenotypic analysis of B-cell lymphoma using a panel of antibodies reactive in routinely fixed wax-embedded tissue. Am J Pathol. 1987 Aug;128(2):225–240. [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Specific identification of intracellular immunoglobulin in paraffin sections of multiple myeloma and macroglobulinemia using an immunoperoxidase technique. Am J Pathol. 1977 Apr;87(1):47–57. [PMC free article] [PubMed] [Google Scholar]
- Pringle J. H., Primrose L., Kind C. N., Talbot I. C., Lauder I. In situ hybridization demonstration of poly-adenylated RNA sequences in formalin-fixed paraffin sections using a biotinylated oligonucleotide poly d(T) probe. J Pathol. 1989 Aug;158(4):279–286. doi: 10.1002/path.1711580403. [DOI] [PubMed] [Google Scholar]
- Seibel N. L., Funa K., Dmitrovsky E., Foss F., Hollis G. F., Kirsch I. R. Application of RNA-RNA tissue in situ hybridization in an analysis of a patient with leukemia. Hum Pathol. 1987 Jan;18(1):3–8. doi: 10.1016/s0046-8177(87)80186-7. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Winberg C. A systematic approach to the immunohistologic classification of lymphoproliferative disorders. Hum Pathol. 1987 Oct;18(10):1051–1062. doi: 10.1016/s0046-8177(87)80222-8. [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Burns J. The demonstration of plasma cells and other immunoglobulin-containing cells in formalin-fixed, paraffin-embedded tissues using peroxidase-labelled antibody. J Clin Pathol. 1974 Jan;27(1):14–20. doi: 10.1136/jcp.27.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. R., Mason D. Y. The immunohistological detection of intracellular immunoglobulin in formalin-paraffin sections from multiple myeloma and related conditions using the immunoperoxidase technique. Clin Exp Immunol. 1974 Nov;18(3):417–429. [PMC free article] [PubMed] [Google Scholar]
- Tubbs R. R., Fishleder A., Weiss R. A., Savage R. A., Sebek B. A., Weick J. K. Immunohistologic cellular phenotypes of lymphoproliferative disorders. Comprehensive evaluation of 564 cases including 257 non-Hodgkin's lymphomas classified by the International Working Formulation. Am J Pathol. 1983 Nov;113(2):207–221. [PMC free article] [PubMed] [Google Scholar]
- Warnke R. A., Rouse R. V. Limitations encountered in the application of tissue section immunodiagnosis to the study of lymphomas and related disorders. Hum Pathol. 1985 Apr;16(4):326–331. doi: 10.1016/s0046-8177(85)80226-4. [DOI] [PubMed] [Google Scholar]