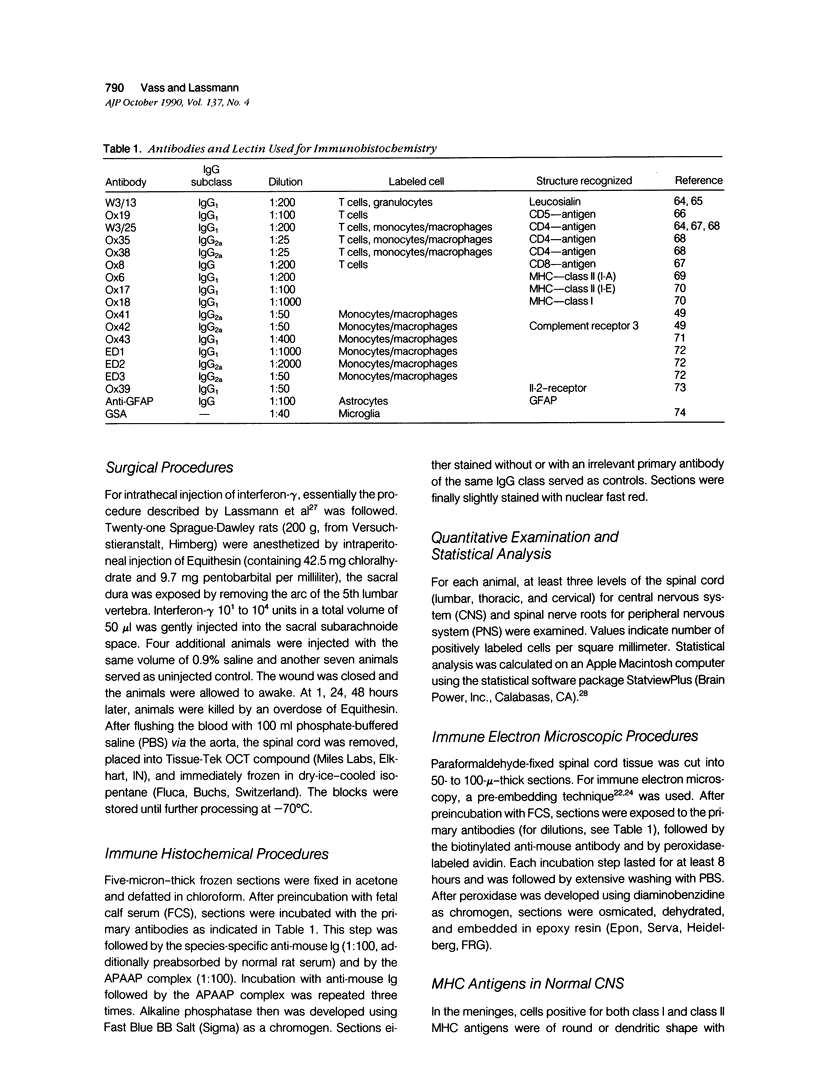
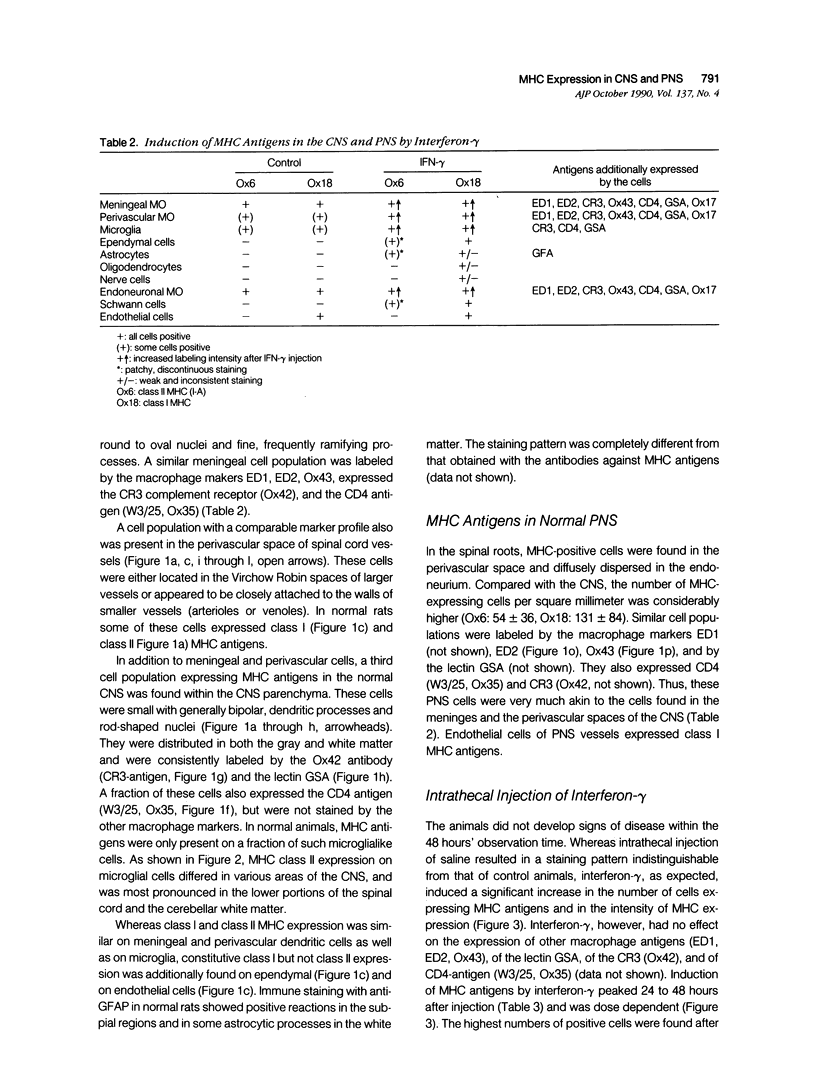
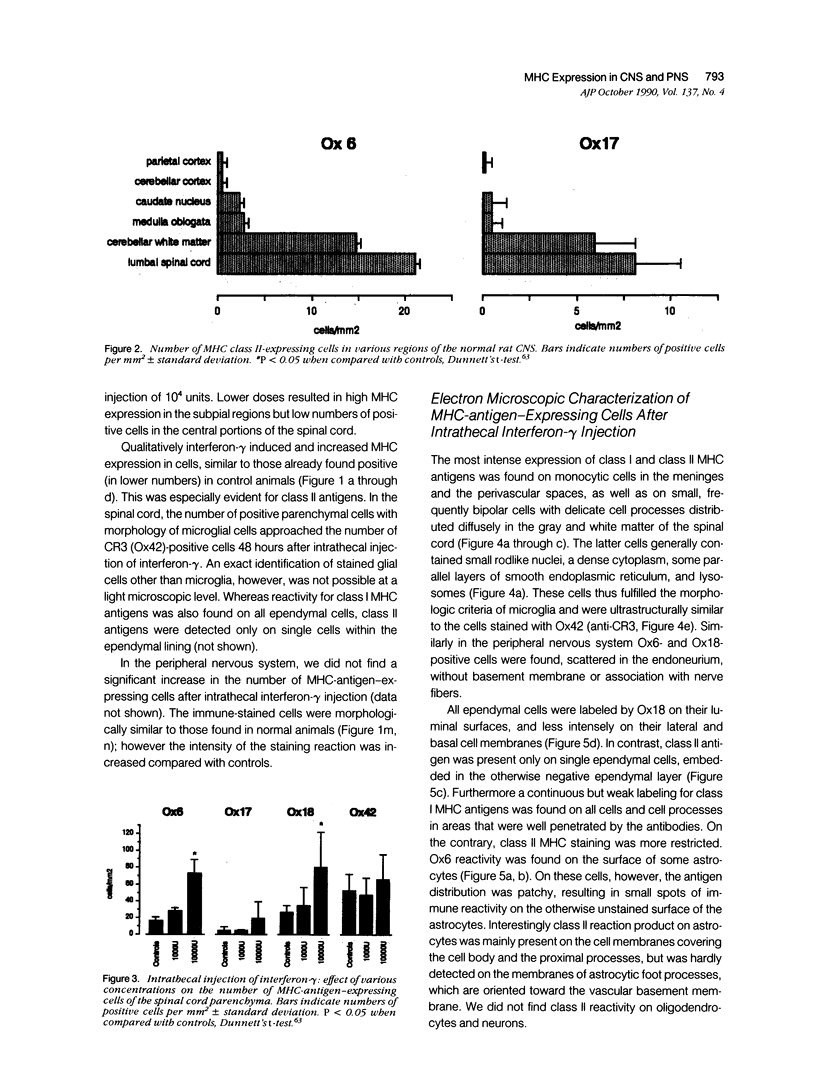
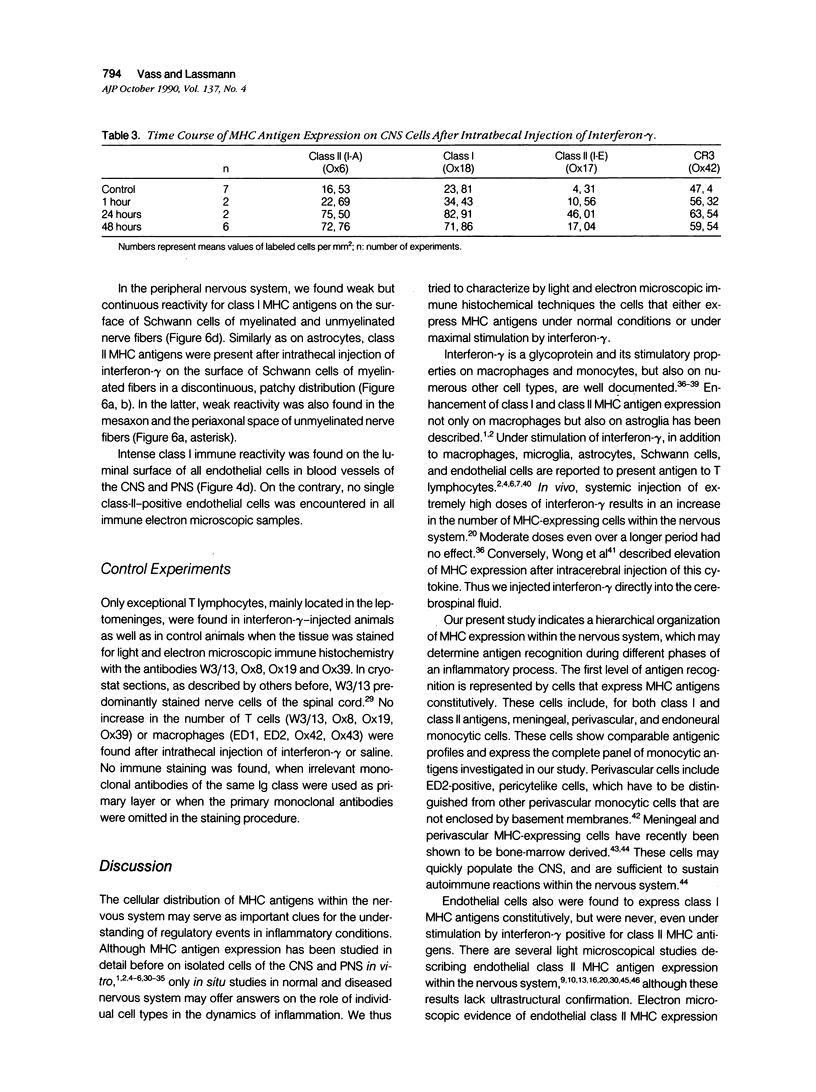
Abstract
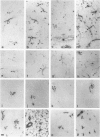
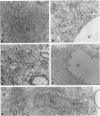
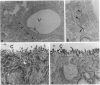
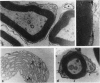
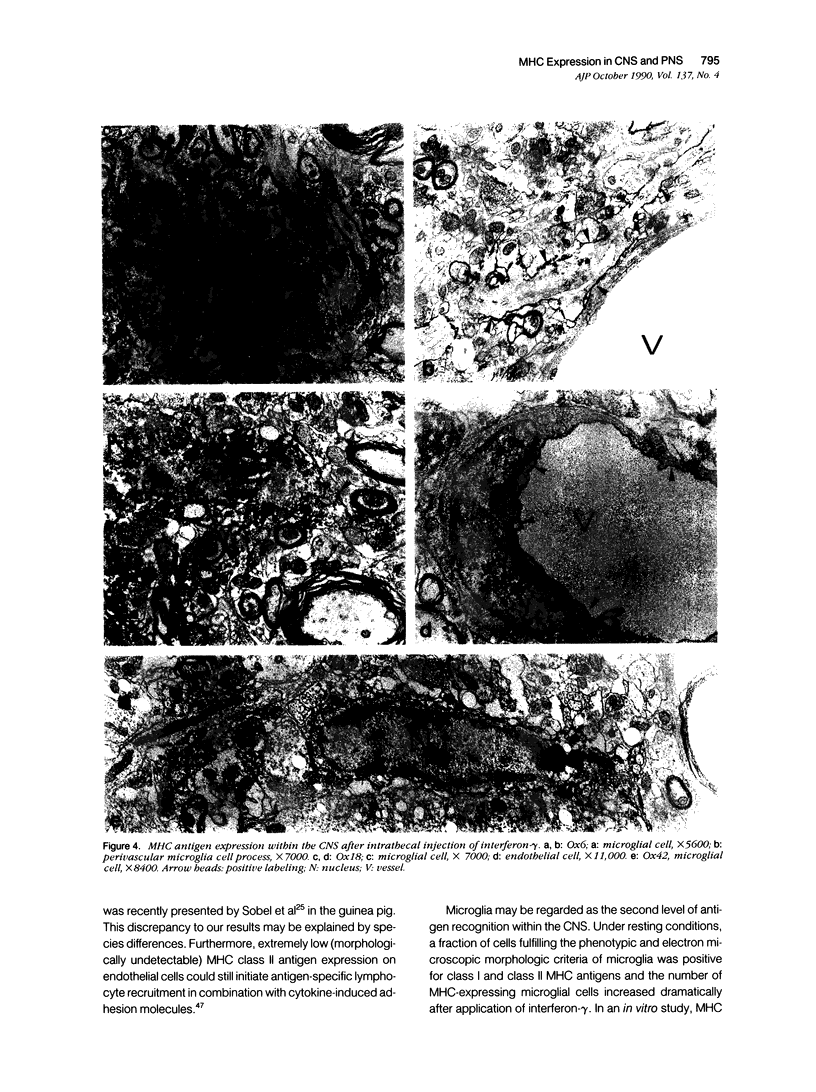
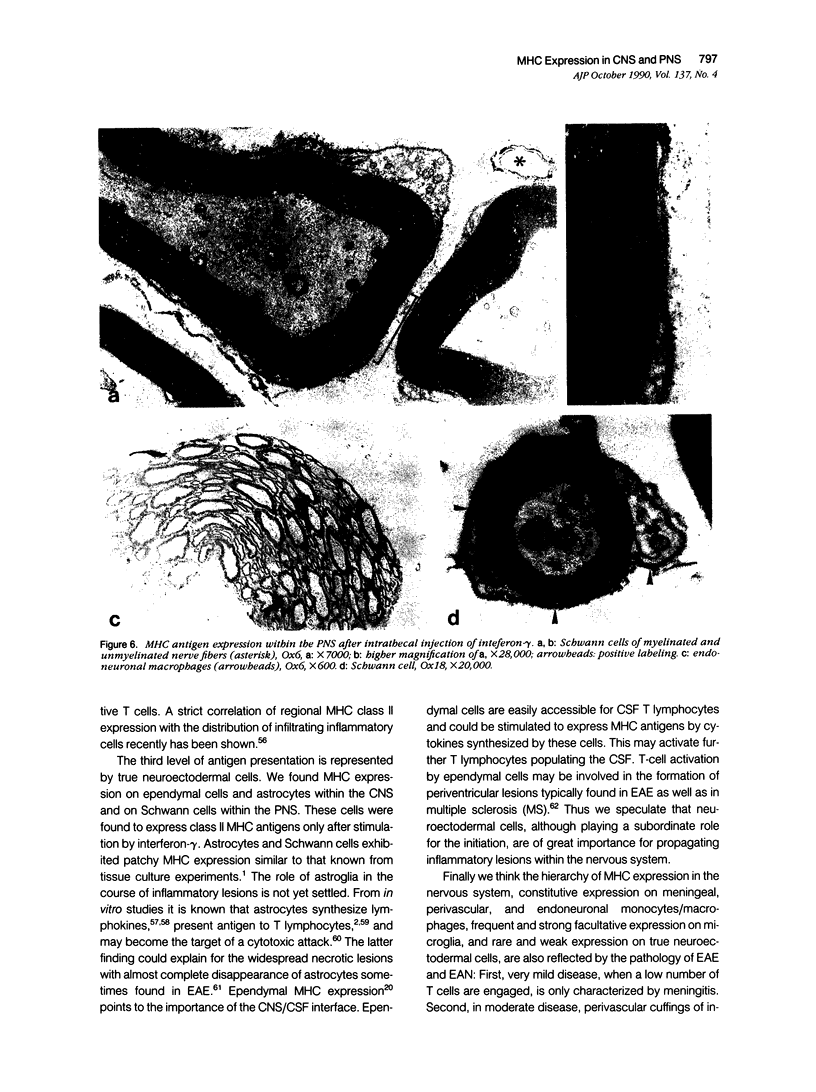
Intrathecal injection of interferon-gamma induced a significant increase of the number of class I and class II major histocompatibility complex (MHC)-expressing cells within the rat nervous system. A progressive appearance of MHC-antigen-positive cells was found by light- and electron microscopic immune histology. The first level comprised cells that constitutively expressed MHC antigens in normal animals (meningeal and endoneural monocytes, some perivascular dendritic cells, and few parenchymal microglia cells, especially in the lumbar spinal cord and in the cerebellar white matter). The second level represented cells readily expressing MHC antigens after stimulation with interferon-gamma (all perivascular, dendritic cells, and microglia). The third level included ependymal cells, astrocytes, and Schwann cells. After stimulation with interferon-gamma, these neuroectodermal cells expressed MHC antigens inconsistently, usually in a low density and patchy distribution. The progressive appearance of MHC antigens may be reflected by the variances of lesional patterns found in experimental allergic encephalomyelitis of different histologic severity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barna B. P., Chou S. M., Jacobs B., Yen-Lieberman B., Ransohoff R. M. Interferon-beta impairs induction of HLA-DR antigen expression in cultured adult human astrocytes. J Neuroimmunol. 1989 Jun;23(1):45–53. doi: 10.1016/0165-5728(89)90072-6. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cadoni A., Zicca A., Mancardi G. L. Schwann cell expression of HLA-DR antigen in peripheral neuropathies. Lancet. 1986 Nov 29;2(8518):1281–1282. [PubMed] [Google Scholar]
- Craggs R. I., Webster H. D. Ia antigens in the normal rat nervous system and in lesions of experimental allergic encephalomyelitis. Acta Neuropathol. 1985;68(4):263–272. doi: 10.1007/BF00690828. [DOI] [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
- De Tribolet N., Hamou M. F., Mach J. P., Carrel S., Schreyer M. Demonstration of HLA-DR antigens in normal human brain. J Neurol Neurosurg Psychiatry. 1984 Apr;47(4):417–418. doi: 10.1136/jnnp.47.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkmans R., Billiau A. Interferon gamma: a master key in the immune system. Curr Opin Immunol. 1988 Dec;1(2):269–274. doi: 10.1016/0952-7915(88)90013-1. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- DuBois J. H., Hammond-Tooke G. D., Cuzner M. L. Expression of major histocompatibility complex antigens in neonate rat primary mixed glial cultures. J Neuroimmunol. 1985 Oct;9(6):363–377. doi: 10.1016/s0165-5728(85)80036-9. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Reading M. C. Macrophage populations associated with multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1987 Nov-Dec;13(6):451–465. doi: 10.1111/j.1365-2990.1987.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Esiri M. M., Reading M. C. Macrophages, lymphocytes and major histocompatibility complex (HLA) class II antigens in adult human sensory and sympathetic ganglia. J Neuroimmunol. 1989 Aug;23(3):187–193. doi: 10.1016/0165-5728(89)90050-7. [DOI] [PubMed] [Google Scholar]
- Fierz W., Endler B., Reske K., Wekerle H., Fontana A. Astrocytes as antigen-presenting cells. I. Induction of Ia antigen expression on astrocytes by T cells via immune interferon and its effect on antigen presentation. J Immunol. 1985 Jun;134(6):3785–3793. [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocytes of the brain synthesize interleukin 3-like factors. J Immunol. 1985 Dec;135(6):4044–4047. [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Frohman E. M., Frohman T. C., Dustin M. L., Vayuvegula B., Choi B., Gupta A., van den Noort S., Gupta S. The induction of intercellular adhesion molecule 1 (ICAM-1) expression on human fetal astrocytes by interferon-gamma, tumor necrosis factor alpha, lymphotoxin, and interleukin-1: relevance to intracerebral antigen presentation. J Neuroimmunol. 1989 Jul;23(2):117–124. doi: 10.1016/0165-5728(89)90030-1. [DOI] [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18(1):155-71, 132-3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Giulian D., Baker T. J., Shih L. C., Lachman L. B. Interleukin 1 of the central nervous system is produced by ameboid microglia. J Exp Med. 1986 Aug 1;164(2):594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber M. B., Streit W. J., Kreutzberg G. W. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989 Jan;22(1):103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- Hayes G. M., Woodroofe M. N., Cuzner M. L. Microglia are the major cell type expressing MHC class II in human white matter. J Neurol Sci. 1987 Aug;80(1):25–37. doi: 10.1016/0022-510x(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988 Jan 15;239(4837):290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hickey W. F., Osborn J. P., Kirby W. M. Expression of Ia molecules by astrocytes during acute experimental allergic encephalomyelitis in the Lewis rat. Cell Immunol. 1985 Apr 1;91(2):528–535. doi: 10.1016/0008-8749(85)90251-5. [DOI] [PubMed] [Google Scholar]
- Hirsch M. R., Wietzerbin J., Pierres M., Goridis C. Expression of Ia antigens by cultured astrocytes treated with gamma-interferon. Neurosci Lett. 1983 Oct 31;41(1-2):199–204. doi: 10.1016/0304-3940(83)90247-1. [DOI] [PubMed] [Google Scholar]
- Hughes C. C., Male D. K., Lantos P. L. Adhesion of lymphocytes to cerebral microvascular cells: effects of interferon-gamma, tumour necrosis factor and interleukin-1. Immunology. 1988 Aug;64(4):677–681. [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen N., Barclay A. N., Willis A. C., Williams A. F. The sequence of rat leukosialin (W3/13 antigen) reveals a molecule with O-linked glycosylation of one third of its extracellular amino acids. EMBO J. 1987 Dec 20;6(13):4029–4034. doi: 10.1002/j.1460-2075.1987.tb02747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert L. E., Paulnock D. M. Differential induction of activation markers in macrophage cell lines by interferon-gamma. Cell Immunol. 1989 May;120(2):401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Hickey W. F. Monoclonal antibody analysis of MHC expression in human brain biopsies: tissue ranging from "histologically normal" to that showing different levels of glial tumor involvement. J Immunol. 1986 Jun 1;136(11):4054–4062. [PubMed] [Google Scholar]
- Lassmann H., Kitz K., Wisniewski H. M. In vivo effect of sera from animals with chronic relapsing experimental allergic encephalomyelitis on central and peripheral myelin. Acta Neuropathol. 1981;55(4):297–306. doi: 10.1007/BF00690994. [DOI] [PubMed] [Google Scholar]
- Lassmann H., Kitz K., Wisniewski H. M. Structural variability of demyelinating lesions in different models of subacute and chronic experimental allergic encephalomyelitis. Acta Neuropathol. 1980;51(3):191–201. doi: 10.1007/BF00687386. [DOI] [PubMed] [Google Scholar]
- Lassmann H., Vass K., Brunner C., Wisniewski H. M. Peripheral nervous system lesions in experimental allergic encephalomyelitis. Ultrastructural distribution of T cells and Ia-antigen. Acta Neuropathol. 1986;69(3-4):193–204. doi: 10.1007/BF00688294. [DOI] [PubMed] [Google Scholar]
- Levine S. Allergic encephalomyelitis: cellular transformation and vascular blockade. J Neuropathol Exp Neurol. 1970 Jan;29(1):6–20. [PubMed] [Google Scholar]
- Losy J., Maehlen J., Olsson T., Kristensson K. Distribution of leukosialin (W3/13)-like immunoreactivity in the rat central nervous system. J Neurocytol. 1989 Feb;18(1):71–76. doi: 10.1007/BF01188425. [DOI] [PubMed] [Google Scholar]
- Maehlen J., Olsson T., Zachau A., Klareskog L., Kristensson K. Local enhancement of major histocompatibility complex (MHC) class I and II expression and cell infiltration in experimental allergic encephalomyelitis around axotomized motor neurons. J Neuroimmunol. 1989 Jul;23(2):125–132. doi: 10.1016/0165-5728(89)90031-3. [DOI] [PubMed] [Google Scholar]
- Maehlen J., Schröder H. D., Klareskog L., Olsson T., Kristensson K. Axotomy induces MHC class I antigen expression on rat nerve cells. Neurosci Lett. 1988 Sep 23;92(1):8–13. doi: 10.1016/0304-3940(88)90733-1. [DOI] [PubMed] [Google Scholar]
- Male D. K., Pryce G., Hughes C. C. Antigen presentation in brain: MHC induction on brain endothelium and astrocytes compared. Immunology. 1987 Mar;60(3):453–459. [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., ter Meulen V., Fontana A. Hyperinducibility of Ia antigen on astrocytes correlates with strain-specific susceptibility to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4219–4223. doi: 10.1073/pnas.84.12.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen-bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987 Dec;17(1):71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- McCarron R. M., Kempski O., Spatz M., McFarlin D. E. Presentation of myelin basic protein by murine cerebral vascular endothelial cells. J Immunol. 1985 May;134(5):3100–3103. [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Yang J., Buciak J., Tourtellotte W. W. Human brain microvascular DR-antigen. J Neurosci Res. 1989 Jul;23(3):337–341. doi: 10.1002/jnr.490230314. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Jefferies W. A., Green J. R., Brandon M. R., Corthesy P., Puklavec M., Williams A. F. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987 Dec;24(12):1281–1290. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Gordon S. Modulation of CD4 antigen on macrophages and microglia in rat brain. J Exp Med. 1987 Oct 1;166(4):1138–1143. doi: 10.1084/jem.166.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. D., Baverstock J., McLeod J. G. Class II antigen expression and inflammatory cells in the Guillain-Barré syndrome. Ann Neurol. 1987 Apr;21(4):337–341. doi: 10.1002/ana.410210404. [DOI] [PubMed] [Google Scholar]
- Pollard J. D., McCombe P. A., Baverstock J., Gatenby P. A., McLeod J. G. Class II antigen expression and T lymphocyte subsets in chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol. 1986 Dec;13(2):123–134. doi: 10.1016/0165-5728(86)90059-7. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. MRC OX-43: a monoclonal antibody which reacts with all vascular endothelium in the rat except that of brain capillaries. Immunology. 1986 Feb;57(2):231–237. [PMC free article] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Sakai K., Tabira T., Endoh M., Steinman L. Ia expression in chronic relapsing experimental allergic encephalomyelitis induced by long-term cultured T cell lines in mice. Lab Invest. 1986 Mar;54(3):345–352. [PubMed] [Google Scholar]
- Sasaki A., Levison S. W., Ting J. P. Comparison and quantitation of Ia antigen expression on cultured macroglia and ameboid microglia from Lewis rat cerebral cortex: analyses and implications. J Neuroimmunol. 1989 Nov;25(1):63–74. doi: 10.1016/0165-5728(89)90087-8. [DOI] [PubMed] [Google Scholar]
- Schelper R. L., Adrian E. K., Jr Monocytes become macrophages; they do not become microglia: a light and electron microscopic autoradiographic study using 125-iododeoxyuridine. J Neuropathol Exp Neurol. 1986 Jan;45(1):1–19. doi: 10.1097/00005072-198601000-00001. [DOI] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sminia T., de Groot C. J., Dijkstra C. D., Koetsier J. C., Polman C. H. Macrophages in the central nervous system of the rat. Immunobiology. 1987 Jan;174(1):43–50. doi: 10.1016/S0171-2985(87)80083-9. [DOI] [PubMed] [Google Scholar]
- Sobel R. A., Blanchette B. W., Bhan A. K., Colvin R. B. The immunopathology of experimental allergic encephalomyelitis. II. Endothelial cell Ia increases prior to inflammatory cell infiltration. J Immunol. 1984 May;132(5):2402–2407. [PubMed] [Google Scholar]
- Sobel R. A., Natale J. M., Schneeberger E. E. The immunopathology of acute experimental allergic encephalomyelitis. IV. An ultrastructural immunocytochemical study of class II major histocompatibility complex molecule (Ia) expression. J Neuropathol Exp Neurol. 1987 May;46(3):239–249. doi: 10.1097/00005072-198705000-00001. [DOI] [PubMed] [Google Scholar]
- Steiniger B., van der Meide P. H. Rat ependyma and microglia cells express class II MHC antigens after intravenous infusion of recombinant gamma interferon. J Neuroimmunol. 1988 Aug;19(1-2):111–118. doi: 10.1016/0165-5728(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Streit W. J., Graeber M. B., Kreutzberg G. W. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989 Aug;105(2):115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit W. J., Kreutzberg G. W. Lectin binding by resting and reactive microglia. J Neurocytol. 1987 Apr;16(2):249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Ting J. P., Shigekawa B. L., Linthicum D. S., Weiner L. P., Frelinger J. A. Expression and synthesis of murine immune response-associated (Ia) antigens by brain cells. Proc Natl Acad Sci U S A. 1981 May;78(5):3170–3174. doi: 10.1073/pnas.78.5.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traugott U., Raine C. S., McFarlin D. E. Acute experimental allergic encephalomyelitis in the mouse: immunopathology of the developing lesion. Cell Immunol. 1985 Mar;91(1):240–254. doi: 10.1016/0008-8749(85)90047-4. [DOI] [PubMed] [Google Scholar]
- Vass K., Lassmann H., Wekerle H., Wisniewski H. M. The distribution of Ia antigen in the lesions of rat acute experimental allergic encephalomyelitis. Acta Neuropathol. 1986;70(2):149–160. doi: 10.1007/BF00691433. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Schwab M., Linington C., Meyermann R. Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol. 1986 Dec;16(12):1551–1557. doi: 10.1002/eji.1830161214. [DOI] [PubMed] [Google Scholar]
- Wilcox C. E., Baker D., Butter C., Willoughby D. A., Turk J. L. Differential expression of guinea pig class II major histocompatibility complex antigens on vascular endothelial cells in vitro and in experimental allergic encephalomyelitis. Cell Immunol. 1989 Apr 15;120(1):82–91. doi: 10.1016/0008-8749(89)90176-7. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]