Abstract
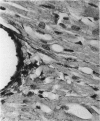
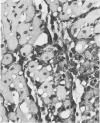
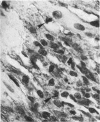
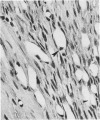
Light microscopic immunohistochemical studies were performed to evaluate the distribution of fibronectin in paraffin sections of p-formaldehyde-fixed normal rat hearts and the hearts of rats that had undergone ligation of the left coronary artery. A peroxidase-labeled antibody technique was used, together with appropriate immunohistochemical control procedures, for the localization of fibronectin in normal hearts and in the hearts of sham-operated animals. Fibronectin was localized in the interstitial space between myocytes, and beneath arterial, venous, and capillary endothelium. At 4 hours after coronary ligation, fibronectin was localized in a patchy fashion in the cytoplasm and interstitial space of some of the myocytes in the area supplied by the ligated vessel. At 24 hours, there was more intense, homogeneous staining in necrotic myocytes in the infarcted area and in the capillary endothelium in the border zone. At 48 hours, the intensity of staining for fibronectin was maximal in and between the necrotic myocytes in the center of the infarct and in proliferating and migrating capillaries and fibroblasts in the border zone. Similar patterns of localization were observed at 3 and 7 days after coronary ligation, but with progressive decreases in the intensity of staining. Two sources of fibronectin appeared to have contributed to these changes: plasma fibronectin diffusing through damaged blood vessels would account for the early staining observed in necrotic myocytes in the center of the infarct, whereas de novo synthesis of fibronectin by connective tissue cells and endothelial cells in sprouting capillaries would be responsible for the subsequent staining observed in viable capillaries in the border zone of the infarct. Known properties of fibronectin in vitro, combined with these in vivo observations, indicate that fibronectin may influence the thrombotic, inflammatory, angiogenic, and fibrotic processes involved in infarct healing.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahumada G. G., Rennard S. I., Figueroa A. A., Silver M. H. Cardiac fibronectin: developmental distribution and quantitative comparison of possible sites of synthesis. J Mol Cell Cardiol. 1981 Jul;13(7):667–678. doi: 10.1016/0022-2828(81)90274-1. [DOI] [PubMed] [Google Scholar]
- Ahumada G. G., Saffitz J. E. Fibronectin in rat heart: a link between cardiac myocytes and collagen. J Histochem Cytochem. 1984 Apr;32(4):383–388. doi: 10.1177/32.4.6707462. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada S. S., Chen W. T., Yamada K. M. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989 Aug;109(2):863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaes N., Boissel J. P. Growth-stimulating effect of catecholamines on rat aortic smooth muscle cells in culture. J Cell Physiol. 1983 Aug;116(2):167–172. doi: 10.1002/jcp.1041160207. [DOI] [PubMed] [Google Scholar]
- Bowersox J. C., Sorgente N. Chemotaxis of aortic endothelial cells in response to fibronectin. Cancer Res. 1982 Jul;42(7):2547–2551. [PubMed] [Google Scholar]
- Casscells W., Bazoberry F., Speir E., Thompson N., Flanders K., Kondaiah P., Ferrans V. J., Epstein S. E., Sporn M. Transforming growth factor-beta 1 in normal heart and in myocardial infarction. Ann N Y Acad Sci. 1990;593:148–160. doi: 10.1111/j.1749-6632.1990.tb16107.x. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Choay J., Lormeau J. C., Petitou M., Sache E., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. II. Evidence for a pentasaccharide sequence that contains a 3-O-sulfate group. J Cell Biol. 1986 May;102(5):1979–1984. doi: 10.1083/jcb.102.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., DellaPelle P., Manseau E., Lanigan J. M., Dvorak H. F., Colvin R. B. Blood vessel fibronectin increases in conjunction with endothelial cell proliferation and capillary ingrowth during wound healing. J Invest Dermatol. 1982 Nov;79(5):269–276. doi: 10.1111/1523-1747.ep12500076. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985 Jun;52(6):611–616. [PubMed] [Google Scholar]
- Connelly C. M., Leppo J. A., Weitzman P. W., Vogel W. M., Apstein C. S. Effect of coronary occlusion and reperfusion on myocardial blood flow during infarct healing. Am J Physiol. 1989 Aug;257(2 Pt 2):H365–H374. doi: 10.1152/ajpheart.1989.257.2.H365. [DOI] [PubMed] [Google Scholar]
- D'Amore P., Shepro D. Stimulation of growth and calcium influx in cultured, bovine, aortic endothelial cells by platelets and vasoactive substances. J Cell Physiol. 1977 Aug;92(2):177–183. doi: 10.1002/jcp.1040920206. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Maclean D., Maroko P. R. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978 Jan;90(1):57–70. [PMC free article] [PubMed] [Google Scholar]
- Form D. M., Pratt B. M., Madri J. A. Endothelial cell proliferation during angiogenesis. In vitro modulation by basement membrane components. Lab Invest. 1986 Nov;55(5):521–530. [PubMed] [Google Scholar]
- Gillery P., Maquart F. X., Borel J. P. Fibronectin dependence of the contraction of collagen lattices by human skin fibroblasts. Exp Cell Res. 1986 Nov;167(1):29–37. doi: 10.1016/0014-4827(86)90201-6. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. The control of mammalian cell proliferation by growth factors, basement lamina, and lipoproteins. J Invest Dermatol. 1983 Jul;81(1 Suppl):40s–50s. doi: 10.1111/1523-1747.ep12540453. [DOI] [PubMed] [Google Scholar]
- Hale S. L., Kloner R. A. Left ventricular topographic alterations in the completely healed rat infarct caused by early and late coronary artery reperfusion. Am Heart J. 1988 Dec;116(6 Pt 1):1508–1513. doi: 10.1016/0002-8703(88)90736-3. [DOI] [PubMed] [Google Scholar]
- Hammerman H., Kloner R. A., Alker K. J., Schoen F. J., Braunwald E. Effects of transient increased afterload during experimentally induced acute myocardial infarction in dogs. Am J Cardiol. 1985 Feb 15;55(5):566–570. doi: 10.1016/0002-9149(85)90248-6. [DOI] [PubMed] [Google Scholar]
- Hasson J. E., Wiebe D. H., Sharefkin J. B., Abbott W. M. Migration of adult human vascular endothelial cells: effect of extracellular matrix proteins. Surgery. 1986 Aug;100(2):384–391. [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Hutchins G. M., Bulkley B. H. Infarct expansion versus extension: two different complications of acute myocardial infarction. Am J Cardiol. 1978 Jun;41(7):1127–1132. doi: 10.1016/0002-9149(78)90869-x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Fibronectins: a family of complex and versatile adhesive glycoproteins derived from a single gene. Harvey Lect. 1985 1986;81:133–152. [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 1989 Jul;109(1):317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E., Madri J. A., Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987 May;23(5):387–394. doi: 10.1007/BF02620997. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Miyazono K., Hellman U., Drexler H., Wernstedt C., Hagiwara K., Usuki K., Takaku F., Risau W., Heldin C. H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989 Apr 13;338(6216):557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- Jaye M., McConathy E., Drohan W., Tong B., Deuel T., Maciag T. Modulation of the sis gene transcript during endothelial cell differentiation in vitro. Science. 1985 May 17;228(4701):882–885. doi: 10.1126/science.3890179. [DOI] [PubMed] [Google Scholar]
- Kent S. P. Diffusion of plasma proteins into cells: a manifestation of cell injury in human myocardial ischemia. Am J Pathol. 1967 Apr;50(4):623–637. [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Sen A., Todaro G. J. Direct association of fibronectin and actin molecules in vitro. J Cell Biol. 1980 Jun;85(3):527–533. doi: 10.1083/jcb.85.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox P., Crooks S., Rimmer C. S. Role of fibronectin in the migration of fibroblasts into plasma clots. J Cell Biol. 1986 Jun;102(6):2318–2323. doi: 10.1083/jcb.102.6.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. A role for fibronectin in the migration of avian precardiac cells. I. Dose-dependent effects of fibronectin antibody. Dev Biol. 1988 Oct;129(2):315–323. doi: 10.1016/0012-1606(88)90378-8. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988 Jul;132(1):18–27. [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Reidy M. A., Kocher O., Bell L. Endothelial cell behavior after denudation injury is modulated by transforming growth factor-beta1 and fibronectin. Lab Invest. 1989 Jun;60(6):755–765. [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernadez A. Electron immunohistochemistry of the extracellular matrix: an overview. Methods Enzymol. 1987;145:78–103. doi: 10.1016/0076-6879(87)45004-0. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt C. H., Lepera R. C., Markwald R. R. Myocardial specificity for initiating endothelial-mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987 Jan;119(1):59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Sjölund M., Palmberg L., Von Euler A. M., Jonzon B., Thyberg J. The calcium antagonist nifedipine inhibits arterial smooth muscle cell proliferation. Atherosclerosis. 1985 Dec;58(1-3):109–122. doi: 10.1016/0021-9150(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Oh E., Pierschbacher M., Ruoslahti E. Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci U S A. 1981 May;78(5):3218–3221. doi: 10.1073/pnas.78.5.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly C. F., Kindy M. S., Brown K. E., Rosenberg R. D., Sonenshein G. E. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J Biol Chem. 1989 Apr 25;264(12):6990–6995. [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W., Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988 Feb;125(2):441–450. doi: 10.1016/0012-1606(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- SELYE H., BAJUSZ E., GRASSO S., MENDELL P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960 Oct;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan L. M., Quigley J. P. An anticatalytic monoclonal antibody to avian plasminogen activator: its effect on behavior of RSV-transformed chick fibroblasts. Cell. 1986 Jun 20;45(6):905–915. doi: 10.1016/0092-8674(86)90565-9. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Aumailley M., Sultan L. H., Martin G. R., Kleinman H. K. Regulation of cell attachment and cell number by fibronectin and laminin. J Cell Physiol. 1986 Jun;127(3):473–479. doi: 10.1002/jcp.1041270318. [DOI] [PubMed] [Google Scholar]
- Teuscher E., Pester E. A possible explanation of mechanisms inducing inhibition of vascularization of tumours by antifibrinolytic drugs--the influence of migratory behaviour of endothelial cells. Biomed Biochim Acta. 1984;43(4):447–456. [PubMed] [Google Scholar]
- Thompson R. W., Orlidge A., D'Amore P. A. Heparin and growth control of vascular cells. Ann N Y Acad Sci. 1989;556:255–267. doi: 10.1111/j.1749-6632.1989.tb22508.x. [DOI] [PubMed] [Google Scholar]
- Ungari S., Katari R. S., Alessandri G., Gullino P. M. Cooperation between fibronectin and heparin in the mobilization of capillary endothelium. Invasion Metastasis. 1985;5(4):193–205. [PubMed] [Google Scholar]
- Williams L. T. Stimulation of paracrine and autocrine pathways of cell proliferation by platelet-derived growth factor. Clin Res. 1988 Jan;36(1):5–10. [PubMed] [Google Scholar]