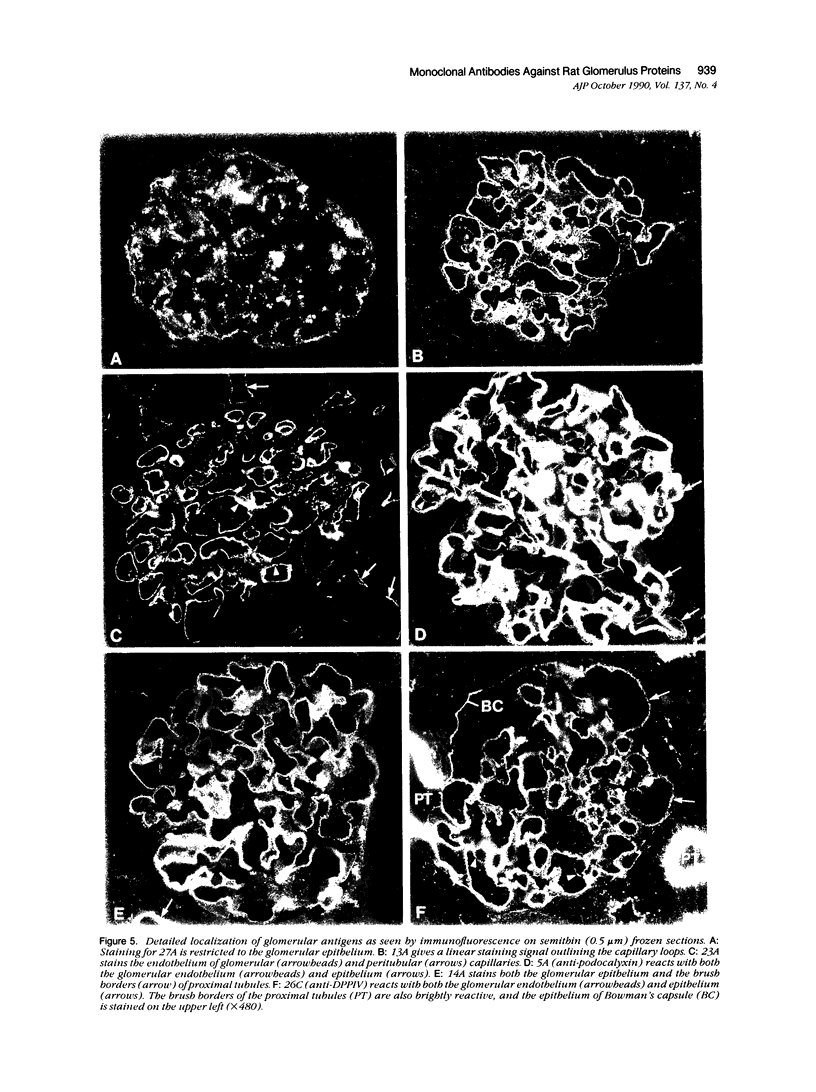
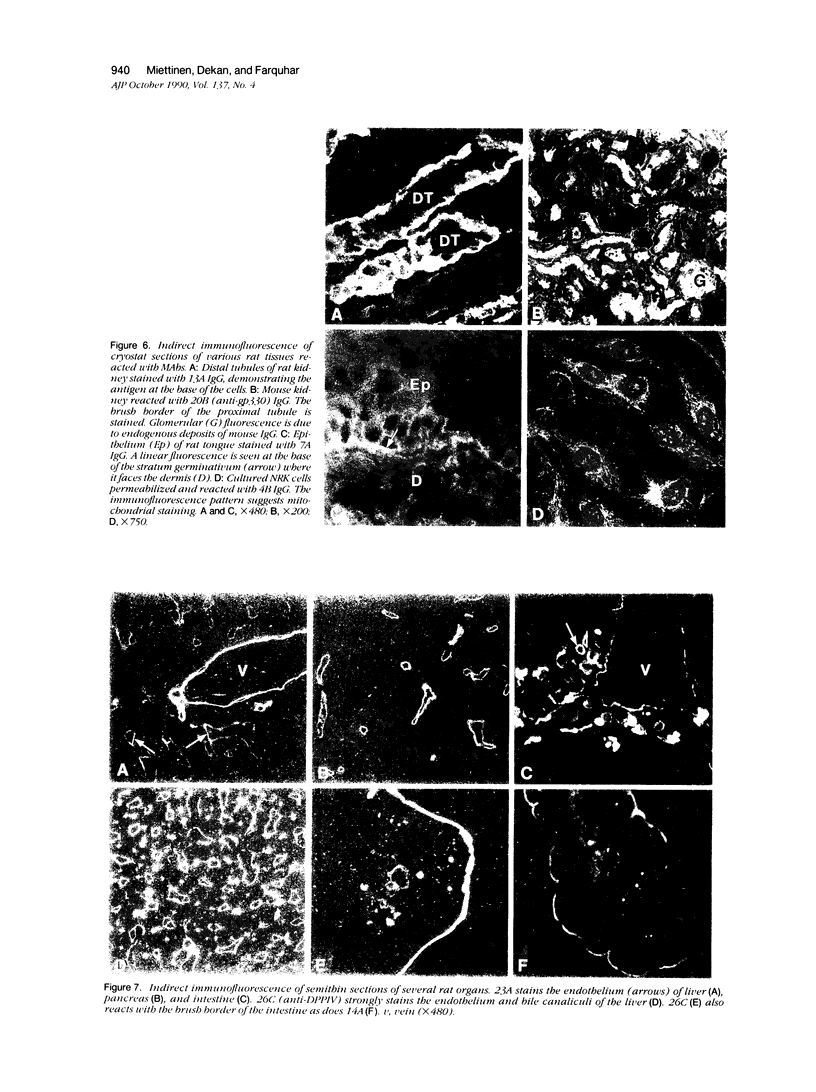
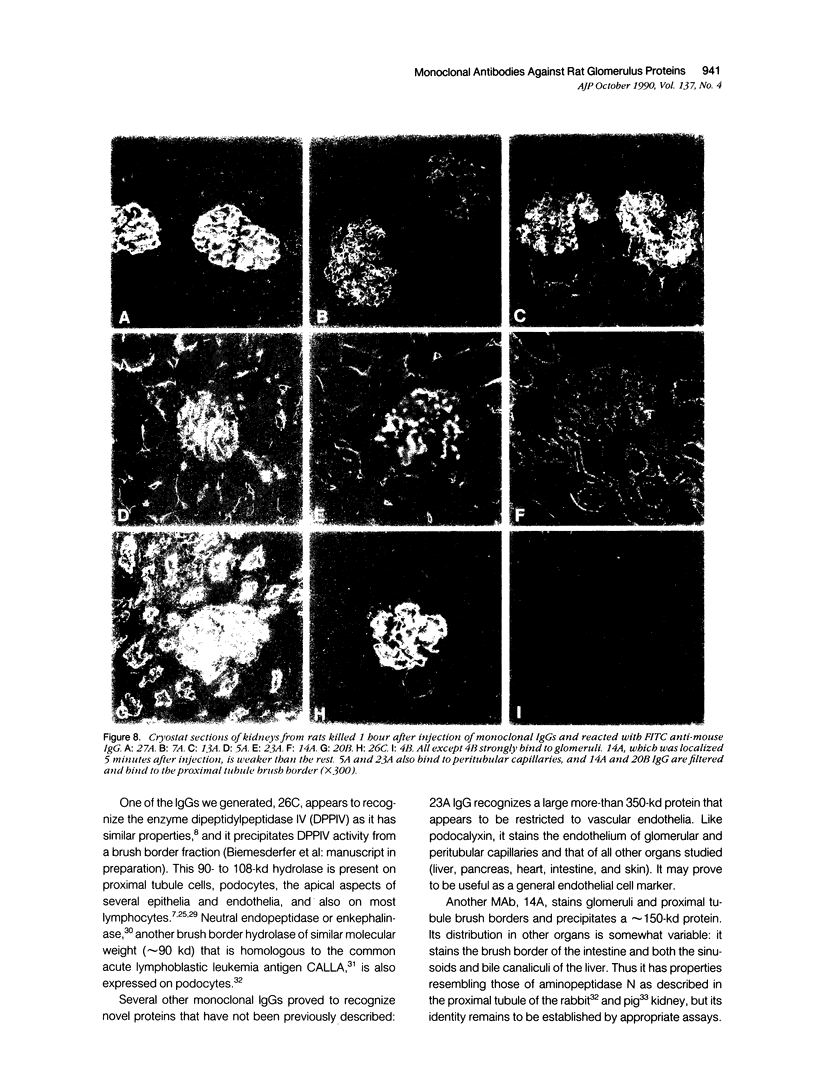
Abstract
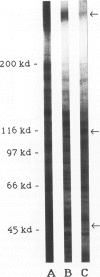
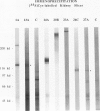
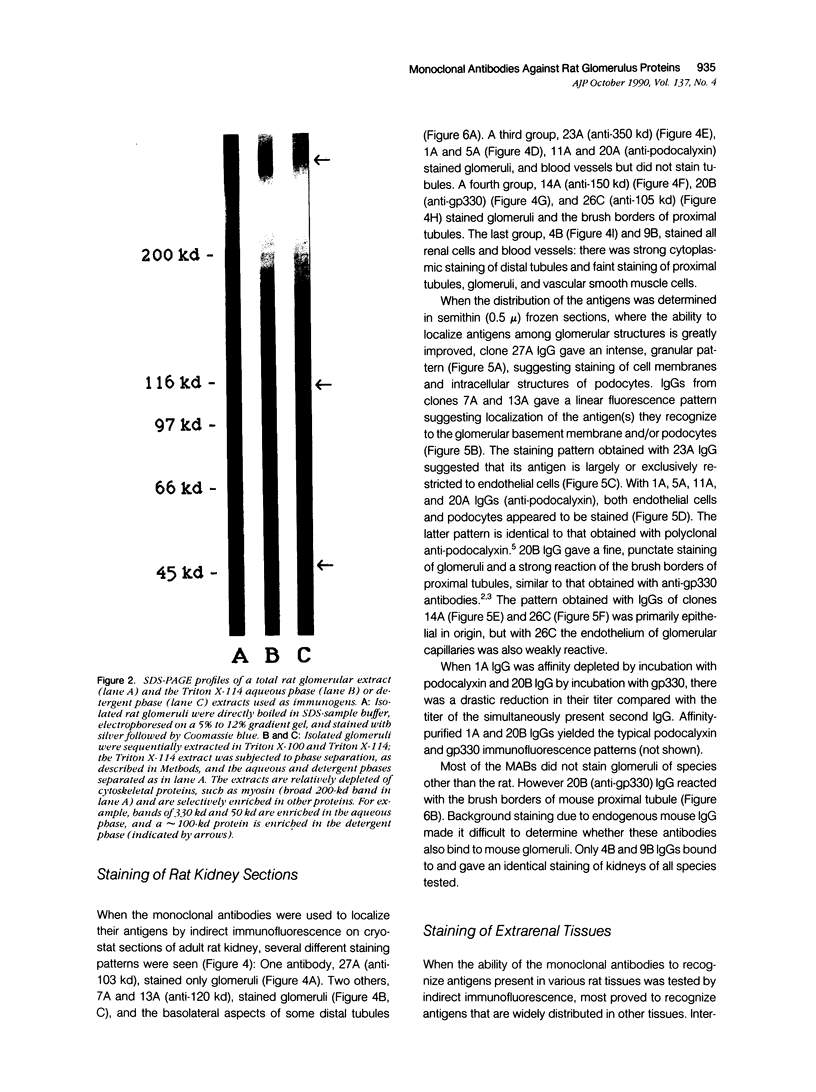
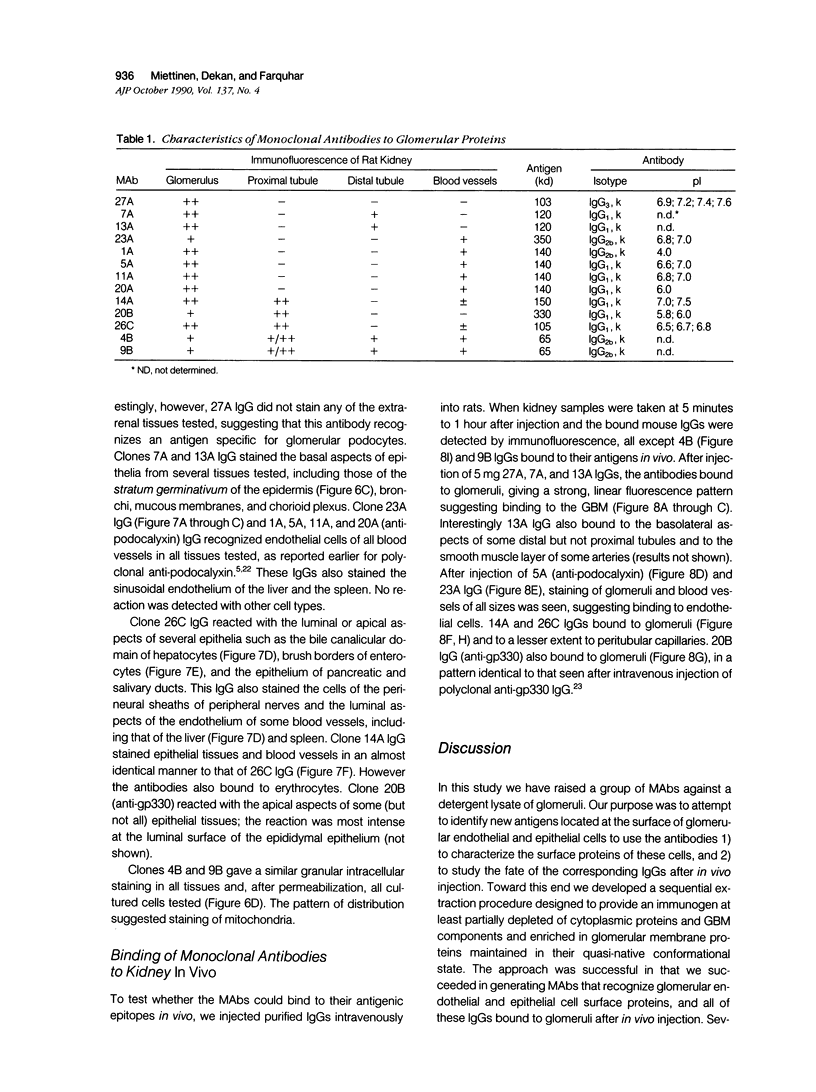
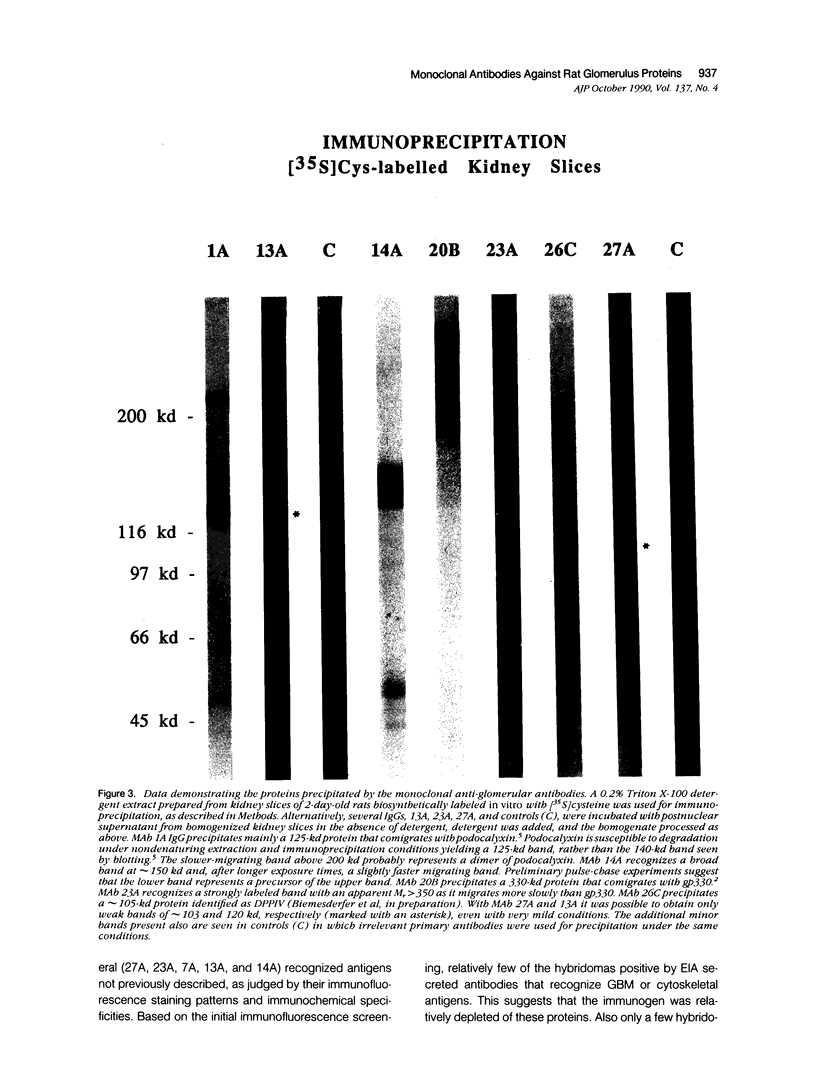
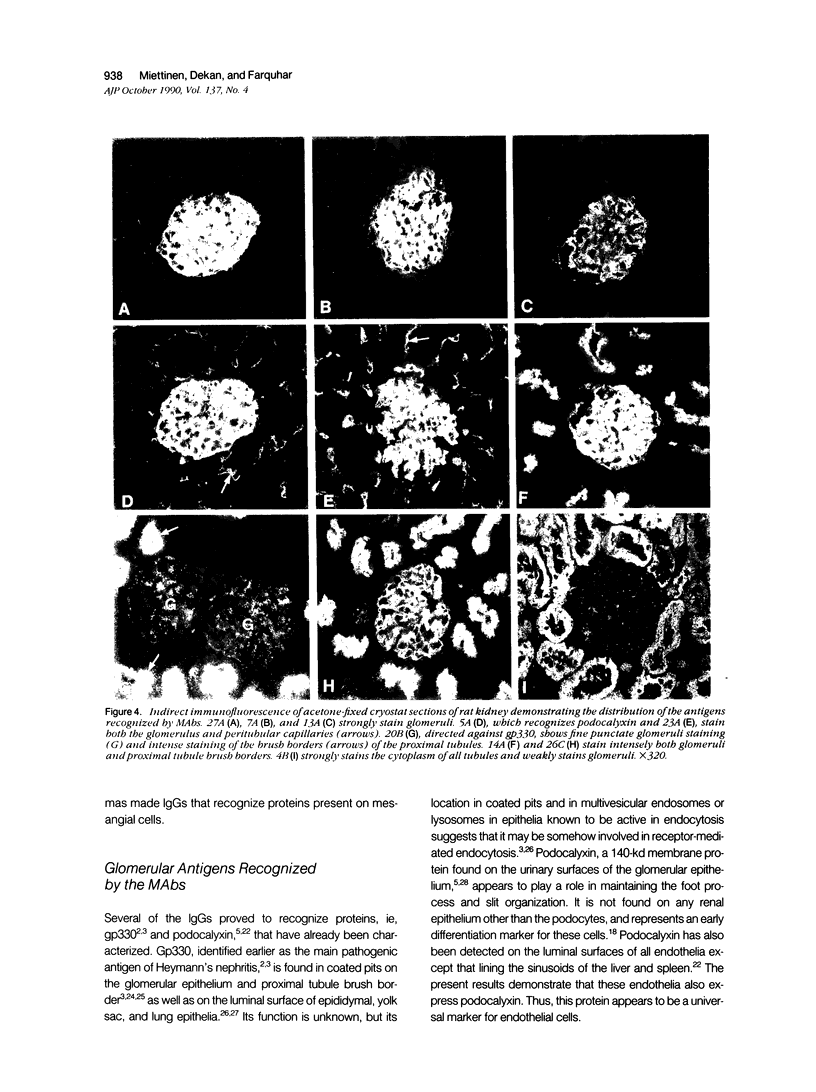
Monoclonal antibodies (MAbs) were generated to detergent-solubilized glomerular extracts to identify new epithelial and endothelial membrane proteins and to study the possible role of the corresponding antigens in the formation of immune deposits. Triton X-114 extracts of isolated glomeruli were subjected to phase separation, and the resultant detergent and aqueous phases were used to immunize mice. Monoclonal antibodies were prepared by standard techniques, and hybridomas secreting antibodies (IgGs) that recognize glomerular cell surface antigens were selected by enzyme immunoassay (EIA) and indirect immunofluorescence. The IgGs of 13 MAbs selected for study recognized antigens of different molecular weights (45-350 kd) by immunoprecipitation and immunoblotting and had different distributions in the glomerulus and in other renal structures by immunofluorescence. Several proved to recognize known antigens--ie, podocalyxin (MAbs 1A, 5A, 11A, and 20A), gp330 (20B), and dipeptidylpeptidase IV (26C). Others recognized antigens not previously characterized that fell into four groups: 1) those that were detected mainly in glomeruli; 2) those present in both glomeruli and peritubular capillaries; 3) those present in both glomeruli and tubule epithelia; and 4) those detected in all these sites. The pattern of glomerular staining also varied, but most of the antigens appeared to be expressed on either the endothelium or the epithelium, or on both. 27A IgG was specific for podocytes and weakly precipitated a 103-kd protein. 7A and 13A IgG precipitated a 120-kd protein and stained glomeruli as well as the basal aspects of distal tubules. 23A IgG recognized a more-than 350-kd antigen that appeared to be specific for endothelial cells in rat kidney and in all other organs studied. 14A IgG precipitated a 150-kd protein and stained glomeruli, proximal tubule brush borders, and endothelial and epithelial cells in rat kidney and in several other organs. 4B and 9B IgG gave a granular cytoplasmic staining in all cells. When injected intravenously into rats, all of the MAbs except 4B and 9B rapidly bound to glomeruli, demonstrating that the respective antigens are exposed at the cell surface and represent potential targets for antibody-mediated immune injury. It is concluded that selective detergent extraction of glomeruli is a useful approach for generation of antibodies that recognize native, nondenatured membrane components of glomerular endothelial and epithelial cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhan A. K., Schneeberger E. E., Baird L. G., Collins A. B., Kamata K., Bradford D., Erikson M. E., McCluskey R. T. Studies with monoclonal antibodies against brush border antigens in Heymann nephritis. Lab Invest. 1985 Oct;53(4):421–432. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Butkowski R. J., Wieslander J., Kleppel M., Michael A. F., Fish A. J. Basement membrane collagen in the kidney: regional localization of novel chains related to collagen IV. Kidney Int. 1989 May;35(5):1195–1202. doi: 10.1038/ki.1989.110. [DOI] [PubMed] [Google Scholar]
- Candelier J. J., Couillin P., Bellon G., Le Pendu J., Eydoux P., Boue A. Demonstration of human kidney differentiation antigens with monoclonal antibodies. J Histochem Cytochem. 1988 Oct;36(10):1255–1262. doi: 10.1177/36.10.3047229. [DOI] [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. I. Renal distribution. Am J Pathol. 1986 Mar;122(3):500–511. [PMC free article] [PubMed] [Google Scholar]
- Chatelet F., Brianti E., Ronco P., Roland J., Verroust P. Ultrastructural localization by monoclonal antibodies of brush border antigens expressed by glomeruli. II. Extrarenal distribution. Am J Pathol. 1986 Mar;122(3):512–519. [PMC free article] [PubMed] [Google Scholar]
- Dekan G., Miettinen A., Schnabel E., Farquhar M. G. Binding of monoclonal antibodies to glomerular endothelium, slit membranes, and epithelium after in vivo injection. Localization of antigens and bound IgGs by immunoelectron microscopy. Am J Pathol. 1990 Oct;137(4):913–927. [PMC free article] [PubMed] [Google Scholar]
- Elovaara I., Seppälä I., Palo J., Sulkava R., Erkinjuntti T. Oligoclonal immunoglobulin bands in cerebrospinal fluid of patients with Alzheimer's disease and vascular dementia. Acta Neurol Scand. 1988 May;77(5):397–401. doi: 10.1111/j.1600-0404.1988.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Falkenberg F. W., Müller E., Riffelmann H. D., Behrendt B., Waks T. The production of monoclonal antibodies against glomerular and other antigens of the human nephron. Ren Physiol. 1981;4(2-3):150–156. doi: 10.1159/000172820. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Atkins R. C. Monoclonal antibodies to human glomerular cells: a marker for glomerular epithelial cells. Nephron. 1983;33(2):83–90. doi: 10.1159/000182918. [DOI] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Lehto V. P., Lehtonen E., Virtanen I. Expression of vimentin and cytokeratin types of intermediate filament proteins in developing and adult human kidneys. Lab Invest. 1984 May;50(5):552–559. [PubMed] [Google Scholar]
- Horvat R., Hovorka A., Dekan G., Poczewski H., Kerjaschki D. Endothelial cell membranes contain podocalyxin--the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol. 1986 Feb;102(2):484–491. doi: 10.1083/jcb.102.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. W., Langlois J. C. Podoendin. A new cell surface protein of the podocyte and endothelium. J Exp Med. 1985 Jul 1;162(1):245–267. doi: 10.1084/jem.162.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. D., Shah V., Merlie J. P., Sanes J. R. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989 Mar 16;338(6212):229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Noronha-Blob L., Sacktor B., Farquhar M. G. Microdomains of distinctive glycoprotein composition in the kidney proximal tubule brush border. J Cell Biol. 1984 Apr;98(4):1505–1513. doi: 10.1083/jcb.98.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Sharkey D. J., Farquhar M. G. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol. 1984 Apr;98(4):1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Sakurai A., Morioka T., Matsui K., Nakamura T., Yakata M., Oite T., Shimizu F. Monoclonal autoantibodies in Heymann nephritis. Clin Exp Immunol. 1986 Jul;65(1):28–33. [PMC free article] [PubMed] [Google Scholar]
- Malfroy B., Schofield P. R., Kuang W. J., Seeburg P. H., Mason A. J., Henzel W. J. Molecular cloning and amino acid sequence of rat enkephalinase. Biochem Biophys Res Commun. 1987 Apr 14;144(1):59–66. doi: 10.1016/s0006-291x(87)80475-8. [DOI] [PubMed] [Google Scholar]
- Mendrick D. L., Rennke H. G., Cotran R. S., Springer T. A., Abbas A. K. Monoclonal antibodies against rat glomerular antigens: production and specificity. Lab Invest. 1983 Jul;49(1):107–117. [PubMed] [Google Scholar]
- Mendrick D. L., Rennke H. G. Induction of proteinuria in the rat by a monoclonal antibody against SGP-115/107. Kidney Int. 1988 Apr;33(4):818–830. doi: 10.1038/ki.1988.73. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Yang J. Y., Falk R. J., Bennington M. J., Scheinman J. I., Vernier R. L., Fish A. J. Monoclonal antibodies to human renal basement membranes: heterogenic and ontogenic changes. Kidney Int. 1983 Jul;24(1):74–86. doi: 10.1038/ki.1983.128. [DOI] [PubMed] [Google Scholar]
- Müller G. A., Müller C. Characterisation of renal antigens on distinct parts of the human nephron by monoclonal antibodies. Klin Wochenschr. 1983 Sep 15;61(18):893–902. doi: 10.1007/BF01537529. [DOI] [PubMed] [Google Scholar]
- Natori Y., Hayakawa I., Shibata S. Identification of gp108, a pathogenic antigen of passive Heymann nephritis, as dipeptidyl peptidase IV. Clin Exp Immunol. 1987 Nov;70(2):434–439. [PMC free article] [PubMed] [Google Scholar]
- Nicol M. J., Miller J. H., Neale T. J. Production of monoclonal antibody probes specific for nonbasement membrane glomerular capillary wall antigens in the rat. Hybridoma. 1987 Aug;6(4):337–347. doi: 10.1089/hyb.1987.6.337. [DOI] [PubMed] [Google Scholar]
- Olsson T., Kostulas V., Link H. Improved detection of oligoclonal IgG in cerebrospinal fluid by isoelectric focusing in agarose, double-antibody peroxidase labeling, and avidin-biotin amplification. Clin Chem. 1984 Jul;30(7):1246–1249. [PubMed] [Google Scholar]
- Orikasa M., Matsui K., Oite T., Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J Immunol. 1988 Aug 1;141(3):807–814. [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohemopoietic differentiation antigens. J Exp Med. 1983 Jan 1;157(1):155–172. doi: 10.1084/jem.157.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt J. L., Michael A. F. Retardation of fading and enhancement of intensity of immunofluorescence by p-phenylenediamine. J Histochem Cytochem. 1983 Jun;31(6):840–842. doi: 10.1177/31.6.6341464. [DOI] [PubMed] [Google Scholar]
- Rolink A. G., Radaszkiewicz T., Melchers F. Monoclonal autoantibodies specific for kidney proximal tubular brush border from mice with experimentally induced chronic graft-versus-host disease. Scand J Immunol. 1988 Jul;28(1):29–41. doi: 10.1111/j.1365-3083.1988.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Ronco P., Geniteau M., Poujeol P., Melcion C., Verroust P., Vandewalle A. Characterization of monoclonal antibodies to rabbit renal cortical cells. Am J Physiol. 1986 Mar;250(3 Pt 1):C506–C516. doi: 10.1152/ajpcell.1986.250.3.C506. [DOI] [PubMed] [Google Scholar]
- Ronco P., Melcion C., Geniteau M., Ronco E., Reininger L., Galceran M., Verroust P. Production and characterization of monoclonal antibodies against rat brush border antigens of the proximal convoluted tubule. Immunology. 1984 Sep;53(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- Sawada H., Stukenbrok H., Kerjaschki D., Farquhar M. G. Epithelial polyanion (podocalyxin) is found on the sides but not the soles of the foot processes of the glomerular epithelium. Am J Pathol. 1986 Nov;125(2):309–318. [PMC free article] [PubMed] [Google Scholar]
- Schnabel E., Dekan G., Miettinen A., Farquhar M. G. Biogenesis of podocalyxin--the major glomerular sialoglycoprotein--in the newborn rat kidney. Eur J Cell Biol. 1989 Apr;48(2):313–326. [PubMed] [Google Scholar]
- Shimizu F., Orikasa M., Sato K., Oite T. Monoclonal antibodies to rat renal antigens. Immunology. 1984 Jun;52(2):319–323. [PMC free article] [PubMed] [Google Scholar]
- Shipp M. A., Vijayaraghavan J., Schmidt E. V., Masteller E. L., D'Adamio L., Hersh L. B., Reinherz E. L. Common acute lymphoblastic leukemia antigen (CALLA) is active neutral endopeptidase 24.11 ("enkephalinase"): direct evidence by cDNA transfection analysis. Proc Natl Acad Sci U S A. 1989 Jan;86(1):297–301. doi: 10.1073/pnas.86.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. R., Kenny A. J. Proteins of the kidney microvillar membrane. Biosynthesis of endopeptidase-24.11, dipeptidylpeptidase IV and aminopeptidases N and A in pig kidney slices. Biochem J. 1984 Dec 1;224(2):549–558. doi: 10.1042/bj2240549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauc M., Chatelet F., Verroust P., Vandewalle A., Poujeol P., Ronco P. Characterization of monoclonal antibodies specific for rabbit renal brush-border hydrolases: application to immunohistological localization. J Histochem Cytochem. 1988 May;36(5):523–532. doi: 10.1177/36.5.2895788. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Application of cryoultramicrotomy to immunocytochemistry. J Microsc. 1986 Aug;143(Pt 2):139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Verroust P., Ronco P. M., Chatelet F. Monoclonal antibodies and identification of glomerular antigens. Kidney Int. 1986 Nov;30(5):649–655. doi: 10.1038/ki.1986.235. [DOI] [PubMed] [Google Scholar]
- Verroust P., Ronco P., Chatelet F. Antigenic targets in membranous glomerulonephritis. Springer Semin Immunopathol. 1987;9(4):341–358. doi: 10.1007/BF00197213. [DOI] [PubMed] [Google Scholar]