Abstract
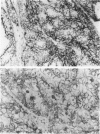
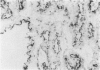
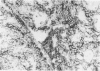
The authors tested frozen sections from 28 renal cell carcinomas (RCC)--21 clear, 1 eosinophilic, 4 basophilic, and 2 spindle-shaped cell type--with monoclonal antibodies (MAb) reacting against cytokeratin, vimentin, CD24, CALLA/CD10, villin, CD26, and HLA class I and class II molecules. These molecules are markers of specific segments of the mature kidney, and their loss or acquisition reflects the different steps of human nephrogenesis. KI67 MAb was used to evaluate cell-proliferating activity. All RCC cases expressed cytokeratin. Coexpression of vimentin was observed in 21 of 28 cases. Whether of clear or chromophilic type, all tumoral cells strongly expressed CD24 molecule, present on primitive blastema cells. All clear-type RCCs expressed CALLA/CD10 and 60% were also villin positive; some were faintly positive for CD26. CALLA, villin, and CD26 were not detected in basophilic cell type. HLA class I molecules were variably expressed in almost all cases, but HLA class II were never detected on tumoral cells. Except for the spindle-shaped population, cell-proliferating activity was low. These results favor the hypothesis that RCCs derive from cells that have 'recovered' the different options of metanephric differentiation. Clear cells show evidence of maturation toward proximal type, while basophilic cells do not. It would be of interest to evaluate the usefulness of serum measurements of villin and/or CALLA as markers in clear cell-type RCC.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andy R. J., Finstad C. L., Old L. J., Lloyd K. O., Kornfeld R. The antigen identified by a mouse monoclonal antibody raised against human renal cancer cells is the adenosine deaminase binding protein. J Biol Chem. 1984 Oct 25;259(20):12844–12849. [PubMed] [Google Scholar]
- Bander N. H. Monoclonal antibodies in urologic oncology. Cancer. 1987 Aug 1;60(3 Suppl):658–667. doi: 10.1002/1097-0142(19870801)60:3+<658::aid-cncr2820601536>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bennington J. L. Proceedings: Cancer of the kidney--etiology, epidemiology, and pathology. Cancer. 1973 Nov;32(5):1017–1029. doi: 10.1002/1097-0142(197311)32:5<1017::aid-cncr2820320501>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Borowitz M. J., Weiss M. A., Bossen E. H., Metzgar R. S. Characterization of renal neoplasms with monoclonal antibodies to leukocyte differentiation antigens. Cancer. 1986 Jan 15;57(2):251–256. doi: 10.1002/1097-0142(19860115)57:2<251::aid-cncr2820570211>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Borthwick G. M., Hughes L., Holmes C. H., Davis S. J., Stirrat G. M. Expression of class I and II major histocompatibility complex antigens in Wilms' tumour and normal developing human kidney. Br J Cancer. 1988 Dec;58(6):753–761. doi: 10.1038/bjc.1988.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselitz J., Osborn M., Wustrow J., Seifert G., Weber K. The expression of different intermediate-sized filaments in human salivary glands and their tumours. Pathol Res Pract. 1982;175(2-3):266–278. doi: 10.1016/S0344-0338(82)80113-1. [DOI] [PubMed] [Google Scholar]
- Cohen C., McCue P. A., Derose P. B. Histogenesis of renal cell carcinoma and renal oncocytoma. An immunohistochemical study. Cancer. 1988 Nov 1;62(9):1946–1951. doi: 10.1002/1097-0142(19881101)62:9<1946::aid-cncr2820620913>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Kerjaschki D., Louvard D. Cytoskeleton organization and submembranous interactions in intestinal and renal brush borders. Kidney Int. 1988 Sep;34(3):309–320. doi: 10.1038/ki.1988.183. [DOI] [PubMed] [Google Scholar]
- Donhuijsen K., Schulz S. Prognostic significance of vimentin positivity in formalin-fixed renal cell carcinomas. Pathol Res Pract. 1989 Mar;184(3):287–291. doi: 10.1016/S0344-0338(89)80088-3. [DOI] [PubMed] [Google Scholar]
- Droz D., Rousseau-Merck M. F., Jaubert F., Diebold N., Nezelof C., Adafer E., Mouly H. Cell differentiation in Wilms' tumor (nephroblastoma): an immunohistochemical study. Hum Pathol. 1990 May;21(5):536–544. doi: 10.1016/0046-8177(90)90011-s. [DOI] [PubMed] [Google Scholar]
- Dudouet B., Jacob L., Beuzeboc P., Magdelenat H., Robine S., Chapuis Y., Christoforov B., Cremer G. A., Pouillard P., Bonnichon P. Presence of villin, a tissue-specific cytoskeletal protein, in sera of patients and an initial clinical evaluation of its value for the diagnosis and follow-up of colorectal cancers. Cancer Res. 1990 Jan 15;50(2):438–443. [PubMed] [Google Scholar]
- Dudouet B., Robine S., Huet C., Sahuquillo-Merino C., Blair L., Coudrier E., Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29-18 clones. J Cell Biol. 1987 Jul;105(1):359–369. doi: 10.1083/jcb.105.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnill M. S., Millard P. R., Oliver D. Acquired cystic disease of the kidneys: a hazard of long-term intermittent maintenance haemodialysis. J Clin Pathol. 1977 Sep;30(9):868–877. doi: 10.1136/jcp.30.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitz W. F., Karthaus H. F., Beck H. L., Romijn C., van der Meyden A. P., Debruyne F. M., Vooijs G. P., Ramaekers F. C. Tissue-specific markers in flow cytometry of urological cancers. II. Cytokeratin and vimentin in renal-cell tumors. Int J Cancer. 1986 Feb 15;37(2):201–207. doi: 10.1002/ijc.2910370206. [DOI] [PubMed] [Google Scholar]
- Finstad C. L., Cordon-Cardo C., Bander N. H., Whitmore W. F., Melamed M. R., Old L. J. Specificity analysis of mouse monoclonal antibodies defining cell surface antigens of human renal cancer. Proc Natl Acad Sci U S A. 1985 May;82(9):2955–2959. doi: 10.1073/pnas.82.9.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S., Lindop G. B., Gibson A. A. The distribution of epithelial membrane antigen in the kidney and its tumours. Histopathology. 1985 Jul;9(7):729–739. doi: 10.1111/j.1365-2559.1985.tb02859.x. [DOI] [PubMed] [Google Scholar]
- Fleming S., Matthews T. J. Renal tubular antigens in regenerative epithelium and renal carcinoma. Br J Urol. 1987 Aug;60(2):103–109. doi: 10.1111/j.1464-410x.1987.tb04942.x. [DOI] [PubMed] [Google Scholar]
- Fleming S., Symes C. E. The distribution of cytokeratin antigens in the kidney and in renal tumours. Histopathology. 1987 Feb;11(2):157–170. doi: 10.1111/j.1365-2559.1987.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Fowler J. E., Jr, Frierson H. F., Jr, Mills S. E., Mariano M. Serum antibody against Tamm-Horsfall protein in patients with renal cell carcinoma. Cancer. 1987 Jun 1;59(11):1923–1926. doi: 10.1002/1097-0142(19870601)59:11<1923::aid-cncr2820591114>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. II. Distribution of filament proteins in normal human tissues. Am J Pathol. 1984 Feb;114(2):309–321. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. III. Analysis of tumors. Am J Clin Pathol. 1985 Oct;84(4):413–424. doi: 10.1093/ajcp/84.4.413. [DOI] [PubMed] [Google Scholar]
- Grassby P. F., Arch J. R., Wilson C., Broadley K. J. Beta-adrenoceptor sensitivity of brown and white adipocytes after chronic pretreatment of rats with reserpine. Biochem Pharmacol. 1987 Jan 1;36(1):155–162. doi: 10.1016/0006-2952(87)90393-5. [DOI] [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Helmchen U., Osborn M. Villin--a marker of brush border differentiation and cellular origin in human renal cell carcinoma. Am J Pathol. 1986 Aug;124(2):294–302. [PMC free article] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Paasivuo R., Lehto V. P., Linder E., Alfthan O., Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983 Sep;49(3):317–326. [PubMed] [Google Scholar]
- Hughson M. D., Buchwald D., Fox M. Renal neoplasia and acquired cystic kidney disease in patients receiving long-term dialysis. Arch Pathol Lab Med. 1986 Jul;110(7):592–601. [PubMed] [Google Scholar]
- Jongeneel C. V., Quackenbush E. J., Ronco P., Verroust P., Carrel S., Letarte M. Common acute lymphoblastic leukemia antigen expressed on leukemia and melanoma cell lines has neutral endopeptidase activity. J Clin Invest. 1989 Feb;83(2):713–717. doi: 10.1172/JCI113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quackenbush E. J., Jongeneel C. V., McInnes R. R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988 Oct 1;168(4):1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvard D. The function of the major cytoskeletal components of the brush border. Curr Opin Cell Biol. 1989 Feb;1(1):51–57. doi: 10.1016/s0955-0674(89)80036-5. [DOI] [PubMed] [Google Scholar]
- McNutt M. A., Bolen J. W., Gown A. M., Hammar S. P., Vogel A. M. Coexpression of intermediate filaments in human epithelial neoplasms. Ultrastruct Pathol. 1985;9(1-2):31–43. doi: 10.3109/01913128509055483. [DOI] [PubMed] [Google Scholar]
- Mechtersheimer G., Möller P. Expression of the common acute lymphoblastic leukemia antigen (CD10) in mesenchymal tumors. Am J Pathol. 1989 May;134(5):961–965. [PMC free article] [PubMed] [Google Scholar]
- Medeiros L. J., Michie S. A., Johnson D. E., Warnke R. A., Weiss L. M. An immunoperoxidase study of renal cell carcinomas: correlation with nuclear grade, cell type, and histologic pattern. Hum Pathol. 1988 Aug;19(8):980–987. doi: 10.1016/s0046-8177(88)80016-9. [DOI] [PubMed] [Google Scholar]
- Mounier F., Foidart J. M., Gubler M. C. Distribution of extracellular matrix glycoproteins during normal development of human kidney. An immunohistochemical study. Lab Invest. 1986 Apr;54(4):394–401. [PubMed] [Google Scholar]
- Natali P. G., Bigotti A., Nicotra M. R., Viora M., Manfredi D., Ferrone S. Distribution of human Class I (HLA-A,B,C) histocompatibility antigens in normal and malignant tissues of nonlymphoid origin. Cancer Res. 1984 Oct;44(10):4679–4687. [PubMed] [Google Scholar]
- Oosterwijk E., Ruiter D. J., Wakka J. C., Huiskens-van der Meij J. W., Jonas U., Fleuren G. J., Zwartendijk J., Hoedemaeker P., Warnaar S. O. Immunohistochemical analysis of monoclonal antibodies to renal antigens. Application in the diagnosis of renal cell carcinoma. Am J Pathol. 1986 May;123(2):301–309. [PMC free article] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Pitz S., Moll R., Störkel S., Thoenes W. Expression of intermediate filament proteins in subtypes of renal cell carcinomas and in renal oncocytomas. Distinction of two classes of renal cell tumors. Lab Invest. 1987 Jun;56(6):642–653. [PubMed] [Google Scholar]
- Platt J. L., LeBien T. W., Michael A. F. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohemopoietic differentiation antigens. J Exp Med. 1983 Jan 1;157(1):155–172. doi: 10.1084/jem.157.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers F. C., Haag D., Kant A., Moesker O., Jap P. H., Vooijs G. P. Coexpression of keratin- and vimentin-type intermediate filaments in human metastatic carcinoma cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2618–2622. doi: 10.1073/pnas.80.9.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine S., Huet C., Moll R., Sahuquillo-Merino C., Coudrier E., Zweibaum A., Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci U S A. 1985 Dec;82(24):8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiter D. J., Bergman W., Welvaart K., Scheffer E., van Vloten W. A., Russo C., Ferrone S. Immunohistochemical analysis of malignant melanomas and nevocellular nevi with monoclonal antibodies to distinct monomorphic determinants of HLA antigens. Cancer Res. 1984 Sep;44(9):3930–3935. [PubMed] [Google Scholar]
- Smith M. E., Bodmer W. F., Bodmer J. G. Selective loss of HLA-A,B,C locus products in colorectal adenocarcinoma. Lancet. 1988 Apr 9;1(8589):823–824. doi: 10.1016/s0140-6736(88)91682-0. [DOI] [PubMed] [Google Scholar]
- Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- Thoenes W., Störkel S., Rumpelt H. J. Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnostics. Pathol Res Pract. 1986 May;181(2):125–143. doi: 10.1016/S0344-0338(86)80001-2. [DOI] [PubMed] [Google Scholar]
- Ulrich W., Horvat R., Krisch K. Lectin histochemistry of kidney tumours and its pathomorphological relevance. Histopathology. 1985 Oct;9(10):1037–1050. doi: 10.1111/j.1365-2559.1985.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Van Muijen G. N., Ruiter D. J., Warnaar S. O. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987 Oct;57(4):359–369. [PubMed] [Google Scholar]
- Waldherr R., Schwechheimer K. Co-expression of cytokeratin and vimentin intermediate-sized filaments in renal cell carcinomas. Comparative study of the intermediate-sized filament distribution in renal cell carcinomas and normal human kidney. Virchows Arch A Pathol Anat Histopathol. 1985;408(1):15–27. doi: 10.1007/BF00739959. [DOI] [PubMed] [Google Scholar]
- Wallace A. C., Nairn R. C. Renal tubular antigens in kidney tumors. Cancer. 1972 Apr;29(4):977–981. doi: 10.1002/1097-0142(197204)29:4<977::aid-cncr2820290444>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Wick M. R., Cherwitz D. L., McGlennen R. C., Dehner L. P. Adrenocortical carcinoma. An immunohistochemical comparison with renal cell carcinoma. Am J Pathol. 1986 Feb;122(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Yao M., Shuin T., Misaki H., Kubota Y. Enhanced expression of c-myc and epidermal growth factor receptor (C-erbB-1) genes in primary human renal cancer. Cancer Res. 1988 Dec 1;48(23):6753–6757. [PubMed] [Google Scholar]
- Yoshida S. O., Imam A., Olson C. A., Taylor C. R. Proximal renal tubular surface membrane antigens identified in primary and metastatic renal cell carcinomas. Arch Pathol Lab Med. 1986 Sep;110(9):825–832. [PubMed] [Google Scholar]