Abstract
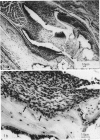
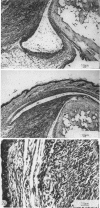
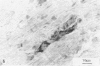
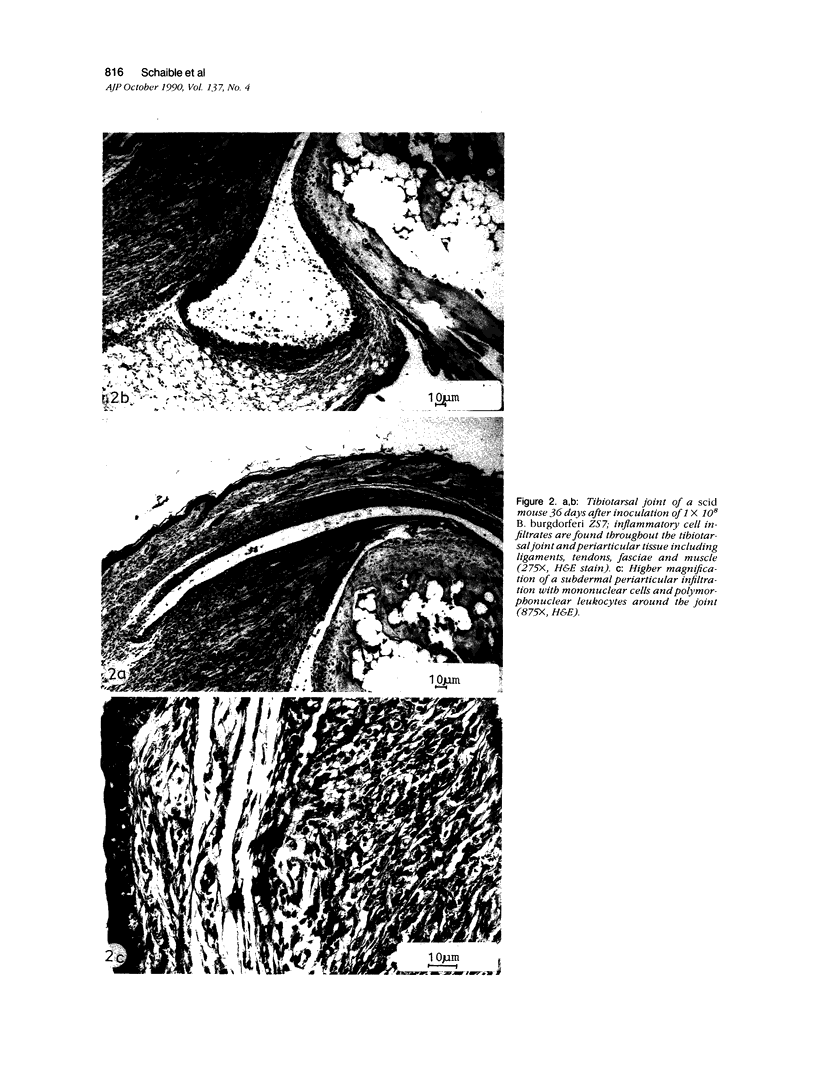
The authors describe the histopathologic evolution of Lyme disease in severe combined immunodeficiency (scid) and normal C.B-17 and C57BL/6 mice inoculated with Borrelia burgdorferi. Starting on day 7 after inoculation, all scid mice infected subcutaneously in the tail with a low-passage European tick isolate of B. burgdorferi had clinical evidence of arthritis characterized by reddening and swelling of tibiotarsal joints. Later on, other joints, ie, metatarsal and ulnacarpal joints were also affected. The infection of scid mice resulted in a persistent spirochetemia and the development of a multisystem disease with chronic progressive inflammation of joints, heart, and liver. Major histopathologic alterations included 1) severe joint lesions, characterized by the presence of hyperplastic inflamed synovial lining cells associated with the erosion and destruction of cartilage and/or bone; 2) pancarditis with infiltrations of mononuclear cells in the endocardium, myocardium, and pericardium; and 3) hepatitis with mononuclear cell infiltrations confined to the portal field and central vein, granulomatous reactions, and eventually the development of liver fibrosis. In addition, smaller more confined lesions were found in kidneys, lung, brain, and striated muscle. The inflammatory infiltrates in the various organs were associated mostly with Mac-1+ cells, largely monocytes and macrophages, as well as some polymorphonuclear leukocytes, but not B and T lymphocytes. Infective spirochetes could be readily isolated from blood and joints and were found at the site of inoculum and the myocardium. In contrast, subcutaneous inoculation of normal C.B-17 or C57BL/6 mice with spirochetes in general did not result in clinical signs of arthritis. Only 10% to 20% of the C57BL/6 mice, but none of the C.B-17 mice, showed clinical evidence of oligoarthritis, which appeared not before day 36 after inoculation. In general, the infection of normal mice resulted in minimal lesions in various organs, and no spirochetes could be visualized or reisolated from their tissues. The data demonstrate that Lyme borreliosis may develop in mice in the absence of detectable specific B and T cells and thus suggest an immunologic control of the disease in this species. The scid mouse model therefore can be used to define the components of the immune system responsible for the suppression and/or the progression of the disease.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas E., Novak S. N., Duray P. H., Steere A. C. Lyme myositis: muscle invasion by Borrelia burgdorferi. Ann Intern Med. 1988 Aug 1;109(3):245–246. doi: 10.7326/0003-4819-109-3-245. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Terwilliger G. A., Duray P. H., Jacoby R. O., Steere A. C. Experimental Lyme arthritis in rats infected with Borrelia burgdorferi. J Infect Dis. 1988 Apr;157(4):842–846. doi: 10.1093/infdis/157.4.842. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Moody K. D., Terwilliger G. A., Jacoby R. O., Steere A. C. An animal model for Lyme arthritis. Ann N Y Acad Sci. 1988;539:264–273. doi: 10.1111/j.1749-6632.1988.tb31860.x. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Garcia-Monco J. C., Deponte P. C. Biological activity of Borrelia burgdorferi antigens. Ann N Y Acad Sci. 1988;539:115–125. doi: 10.1111/j.1749-6632.1988.tb31845.x. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavanet P., Pillon D., Lancon J. P., Waldner-Combernoux A., Maringe E., Portier H. Granulomatous hepatitis associated with Lyme disease. Lancet. 1987 Sep 12;2(8559):623–624. doi: 10.1016/s0140-6736(87)93009-1. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989 May;57(5):1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer R. P., Bosma G. C., Bosma M. J. Severe combined immunodeficiency (SCID) in the mouse. Pathology, reconstitution, neoplasms. Am J Pathol. 1985 Sep;120(3):464–477. [PMC free article] [PubMed] [Google Scholar]
- Dattwyler R. J., Volkman D. J., Halperin J. J., Luft B. J., Thomas J., Golightly M. G. Specific immune responses in Lyme borreliosis. Characterization of T cell and B cell responses to Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:93–102. doi: 10.1111/j.1749-6632.1988.tb31842.x. [DOI] [PubMed] [Google Scholar]
- Deschryver-Kecskemeti K., Bancroft G. J., Bosma G. C., Bosma M. J., Unanue E. R. Pathology of Listeria infection in murine severe combined immunodeficiency. A study by immunohistochemistry and electron microscopy. Lab Invest. 1988 Jun;58(6):698–705. [PubMed] [Google Scholar]
- Duray P. H., Steere A. C. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79. doi: 10.1111/j.1749-6632.1988.tb31839.x. [DOI] [PubMed] [Google Scholar]
- Goellner M. H., Agger W. A., Burgess J. H., Duray P. H. Hepatitis due to recurrent Lyme disease. Ann Intern Med. 1988 May;108(5):707–708. doi: 10.7326/0003-4819-108-5-707. [DOI] [PubMed] [Google Scholar]
- Golightly M., Thomas J., Volkman D., Dattwyler R. Modulation of natural killer cell activity by Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:103–111. doi: 10.1111/j.1749-6632.1988.tb31843.x. [DOI] [PubMed] [Google Scholar]
- Hardin J. A., Steere A. C., Malawista S. E. Immune complexes and the evolution of Lyme arthritis. Dissemination and localization of abnormal C1q binding activity. N Engl J Med. 1979 Dec 20;301(25):1358–1363. doi: 10.1056/NEJM197912203012502. [DOI] [PubMed] [Google Scholar]
- Hejka A., Schmitz J. L., England D. M., Callister S. M., Schell R. F. Histopathology of Lyme arthritis in LSH hamsters. Am J Pathol. 1989 May;134(5):1113–1123. [PMC free article] [PubMed] [Google Scholar]
- Johnson S. E., Klein G. C., Schmid G. P., Bowen G. S., Feeley J. C., Schulze T. Lyme disease: a selective medium for isolation of the suspected etiological agent, a spirochete. J Clin Microbiol. 1984 Jan;19(1):81–82. doi: 10.1128/jcm.19.1.81-82.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston Y. E., Duray P. H., Steere A. C., Kashgarian M., Buza J., Malawista S. E., Askenase P. W. Lyme arthritis. Spirochetes found in synovial microangiopathic lesions. Am J Pathol. 1985 Jan;118(1):26–34. [PMC free article] [PubMed] [Google Scholar]
- Kramer M. D., Fruth U., Simon H. G., Simon M. M. Expression of cytoplasmic granules with T cell-associated serine proteinase-1 activity in Ly-2+(CD8+) T lymphocytes responding to lymphocytic choriomeningitis virus in vivo. Eur J Immunol. 1989 Jan;19(1):151–156. doi: 10.1002/eji.1830190124. [DOI] [PubMed] [Google Scholar]
- O'Sullivan F. X., Fassbender H. G., Gay S., Koopman W. J. Etiopathogenesis of the rheumatoid arthritis-like disease in MRL/l mice. I. The histomorphologic basis of joint destruction. Arthritis Rheum. 1985 May;28(5):529–536. doi: 10.1002/art.1780280511. [DOI] [PubMed] [Google Scholar]
- Reimers C. D., Pongratz D. E., Neubert U., Pilz A., Hübner G., Naegele M., Wilske B., Duray P. H., de Koning J. Myositis caused by Borrelia burgdorferi: report of four cases. J Neurol Sci. 1989 Jun;91(1-2):215–226. doi: 10.1016/0022-510x(89)90089-0. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Museteanu C., Zimmer G., Mossmann H., Simon M. M. The severe combined immunodeficiency (scid) mouse. A laboratory model for the analysis of Lyme arthritis and carditis. J Exp Med. 1989 Oct 1;170(4):1427–1432. doi: 10.1084/jem.170.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J. L., Schell R. F., Hejka A., England D. M., Konick L. Induction of lyme arthritis in LSH hamsters. Infect Immun. 1988 Sep;56(9):2336–2342. doi: 10.1128/iai.56.9.2336-2342.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Duray P. H., Butcher E. C. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988 Apr;31(4):487–495. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Hardin J. A., Ruddy S., Mummaw J. G., Malawista S. E. Lyme arthritis: correlation of serum and cryoglobulin IgM with activity, and serum IgG with remission. Arthritis Rheum. 1979 May;22(5):471–483. doi: 10.1002/art.1780220506. [DOI] [PubMed] [Google Scholar]
- Tanaka A., O'Sullivan F. X., Koopman W. J., Gay S. Etiopathogenesis of rheumatoid arthritis-like disease in MRL/1 mice: II. Ultrastructural basis of joint destruction. J Rheumatol. 1988 Jan;15(1):10–16. [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- de Koning J., Hoogkamp-Korstanje J. A., van der Linde M. R., Crijns H. J. Demonstration of spirochetes in cardiac biopsies of patients with Lyme disease. J Infect Dis. 1989 Jul;160(1):150–153. doi: 10.1093/infdis/160.1.150. [DOI] [PubMed] [Google Scholar]