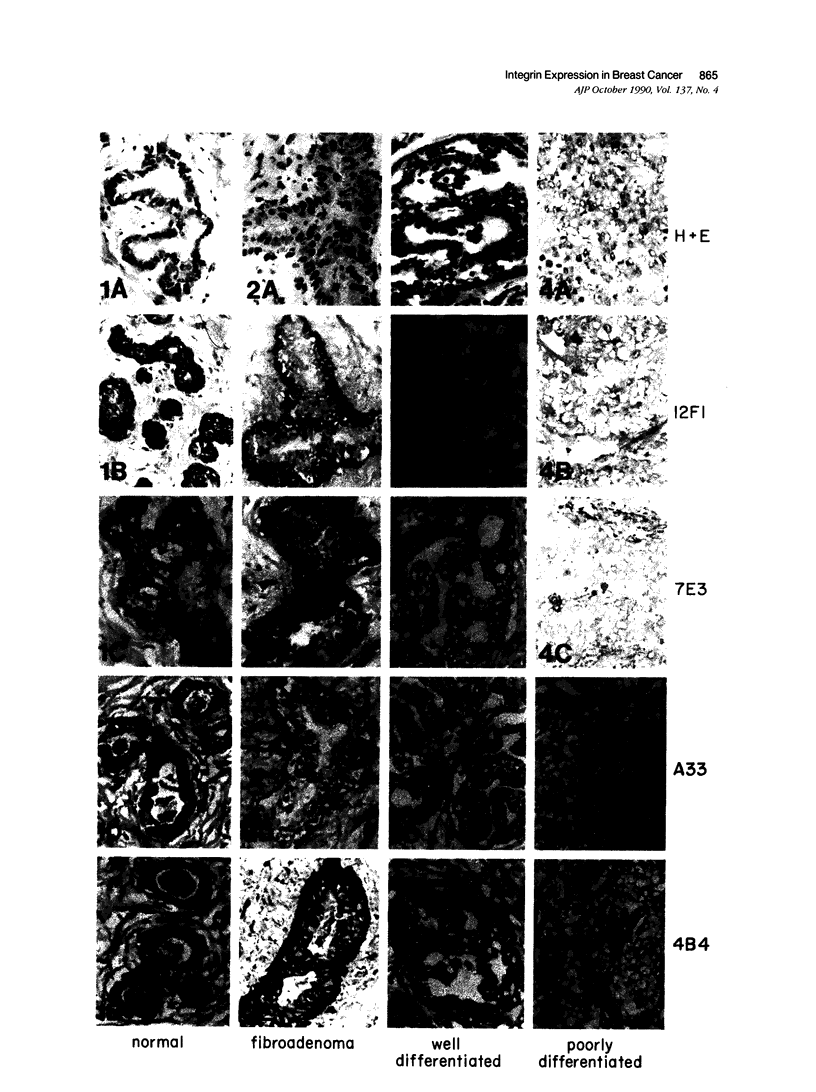
Abstract
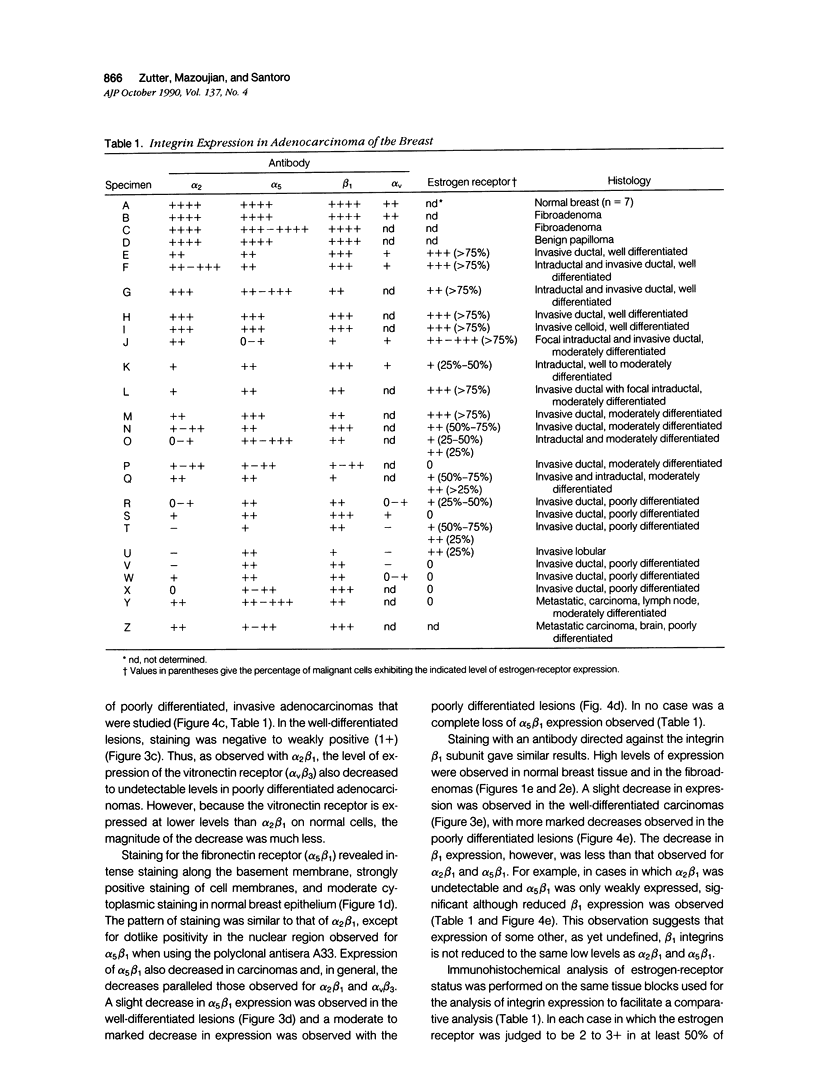
The integrin superfamily represents a major class of receptors mediating cell-substrate adhesion. Our recent study of the tissue distribution of the alpha 2 beta 1 integrin, a cell-surface collagen receptor, revealed that high levels of receptor expression were associated with orderly, regulated epithelial cell proliferation. Those observations prompted the present investigation of alpha 2 beta 1 and other integrins in adenocarcinoma of the breast. The alpha 2 beta 1 integrin was highly expressed on the epithelium of the ducts and ductules of normal breast tissue. Normal or nearly normal levels of the receptor were expressed in fibroadenomas. In contrast, markedly decreased or undetectable alpha 2 beta 1 expression was typical of poorly differentiated adenocarcinomas. Well-differentiated lesions exhibited intermediate levels of expression. Similar, but less extensive, decreases in expression were observed for the alpha 5 beta 1 (fibronectin receptor) and alpha v beta 3 (vitronectin receptor). Significant expression of the beta 1 subunit on even poorly differentiated tumors suggests that the expression of other undefined members of the beta 1 family is not reduced to the same low level as alpha 2 beta 1 and alpha 5 beta 1. Expression of the alpha 2 beta 1 integrin was highly correlated with estrogen-receptor expression. Decreased expression of alpha 2 beta 1 and other integrin adhesive protein receptors probably contributes to the altered adhesive properties of tumor cells characteristic of the malignant phenotype.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Graf J., Kitten G. T., Kleinman H. K., Martin G. R., Veillette A., Lippman M. E. 17 beta-estradiol regulates and v-Ha-ras transfection constitutively enhances MCF7 breast cancer cell interactions with basement membrane. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8182–8186. doi: 10.1073/pnas.83.21.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Mareel M. M., Van Roy F. M., Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989 Jun;108(6):2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger U., Wilson P., McClelland R. A., Davidson J., Coombes R. C. Correlation of immunocytochemically demonstrated estrogen receptor distribution and histopathologic features in primary breast cancer. Hum Pathol. 1987 Dec;18(12):1263–1267. doi: 10.1016/s0046-8177(87)80411-2. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Castronovo V., Taraboletti G., Liotta L. A., Sobel M. E. Modulation of laminin receptor expression by estrogen and progestins in human breast cancer cell lines. J Natl Cancer Inst. 1989 May 10;81(10):781–788. doi: 10.1093/jnci/81.10.781. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Bekeart L. S., Phillips D. R. Platelet glycoprotein IIb-IIIa-like proteins mediate endothelial cell attachment to adhesive proteins and the extracellular matrix. J Biol Chem. 1987 Jul 25;262(21):9935–9938. [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Clark G. M., McGuire W. L. Steroid receptors and other prognostic factors in primary breast cancer. Semin Oncol. 1988 Apr;15(2 Suppl 1):20–25. [PubMed] [Google Scholar]
- Coller B. S. A new murine monoclonal antibody reports an activation-dependent change in the conformation and/or microenvironment of the platelet glycoprotein IIb/IIIa complex. J Clin Invest. 1985 Jul;76(1):101–108. doi: 10.1172/JCI111931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Hemler M. E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. W., Young N. A. Human breast carcinoma cells in continuous culture: a review. Cancer Res. 1978 Nov;38(11 Pt 2):4327–4339. [PubMed] [Google Scholar]
- Hand P. H., Thor A., Schlom J., Rao C. N., Liotta L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985 Jun;45(6):2713–2719. [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G. Cell matrix adhesion-related proteins VLA-1 and VLA-2: regulation of expression on T cells. J Immunol. 1987 May 1;138(9):2941–2948. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Kao R. T., Stern R. Collagenases in human breast carcinoma cell lines. Cancer Res. 1986 Mar;46(3):1349–1354. [PubMed] [Google Scholar]
- Kunicki T. J., Nugent D. J., Staats S. J., Orchekowski R. P., Wayner E. A., Carter W. G. The human fibroblast class II extracellular matrix receptor mediates platelet adhesion to collagen and is identical to the platelet glycoprotein Ia-IIa complex. J Biol Chem. 1988 Apr 5;263(10):4516–4519. [PubMed] [Google Scholar]
- Languino L. R., Gehlsen K. R., Wayner E., Carter W. G., Engvall E., Ruoslahti E. Endothelial cells use alpha 2 beta 1 integrin as a laminin receptor. J Cell Biol. 1989 Nov;109(5):2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millon R., Nicora F., Muller D., Eber M., Klein-Soyer C., Abecassis J. Modulation of human breast cancer cell adhesion by estrogens and antiestrogens. Clin Exp Metastasis. 1989 Jul-Aug;7(4):405–415. doi: 10.1007/BF01753661. [DOI] [PubMed] [Google Scholar]
- Morisset M., Capony F., Rochefort H. The 52-kDa estrogen-induced protein secreted by MCF7 cells is a lysosomal acidic protease. Biochem Biophys Res Commun. 1986 Jul 16;138(1):102–109. doi: 10.1016/0006-291x(86)90252-4. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Hobbs K., Clark G. M. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985 Feb;45(2):584–590. [PubMed] [Google Scholar]
- Peltonen J., Larjava H., Jaakkola S., Gralnick H., Akiyama S. K., Yamada S. S., Yamada K. M., Uitto J. Localization of integrin receptors for fibronectin, collagen, and laminin in human skin. Variable expression in basal and squamous cell carcinomas. J Clin Invest. 1989 Dec;84(6):1916–1923. doi: 10.1172/JCI114379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Bluestein H. G., Woods V. L., Jr Platelet glycoproteins Ia, Ic, and IIa are physicochemically indistinguishable from the very late activation antigens adhesion-related proteins of lymphocytes and other cell types. J Clin Invest. 1988 Feb;81(2):505–513. doi: 10.1172/JCI113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischel K. D., Hemler M. E., Huang C., Bluestein H. G., Woods V. L., Jr Use of the monoclonal antibody 12F1 to characterize the differentiation antigen VLA-2. J Immunol. 1987 Jan 1;138(1):226–233. [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Roman J., LaChance R. M., Broekelmann T. J., Kennedy C. J., Wayner E. A., Carter W. G., McDonald J. A. The fibronectin receptor is organized by extracellular matrix fibronectin: implications for oncogenic transformation and for cell recognition of fibronectin matrices. J Cell Biol. 1989 Jun;108(6):2529–2543. doi: 10.1083/jcb.108.6.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Santoro S. A. Identification of a 160,000 dalton platelet membrane protein that mediates the initial divalent cation-dependent adhesion of platelets to collagen. Cell. 1986 Sep 12;46(6):913–920. doi: 10.1016/0092-8674(86)90073-5. [DOI] [PubMed] [Google Scholar]
- Santoro S. A., Rajpara S. M., Staatz W. D., Woods V. L., Jr Isolation and characterization of a platelet surface collagen binding complex related to VLA-2. Biochem Biophys Res Commun. 1988 May 31;153(1):217–223. doi: 10.1016/s0006-291x(88)81211-7. [DOI] [PubMed] [Google Scholar]
- Shafie S. M., Liotta L. A. Formation of metastasis by human breast carcinoma cells (MCF-7) in nude mice. Cancer Lett. 1980 Dec;11(2):81–87. doi: 10.1016/0304-3835(80)90097-x. [DOI] [PubMed] [Google Scholar]
- Staatz W. D., Rajpara S. M., Wayner E. A., Carter W. G., Santoro S. A. The membrane glycoprotein Ia-IIa (VLA-2) complex mediates the Mg++-dependent adhesion of platelets to collagen. J Cell Biol. 1989 May;108(5):1917–1924. doi: 10.1083/jcb.108.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Wayner E. A., Carter W. G., Hemler M. E. Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem. 1988 Aug;37(4):385–393. doi: 10.1002/jcb.240370406. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley B. R., May F. E. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res. 1987 May 11;15(9):3773–3786. doi: 10.1093/nar/15.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Zutter M. M., Santoro S. A. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am J Pathol. 1990 Jul;137(1):113–120. [PMC free article] [PubMed] [Google Scholar]