Abstract
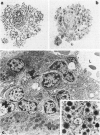
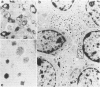
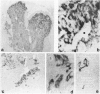
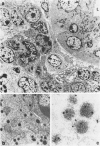
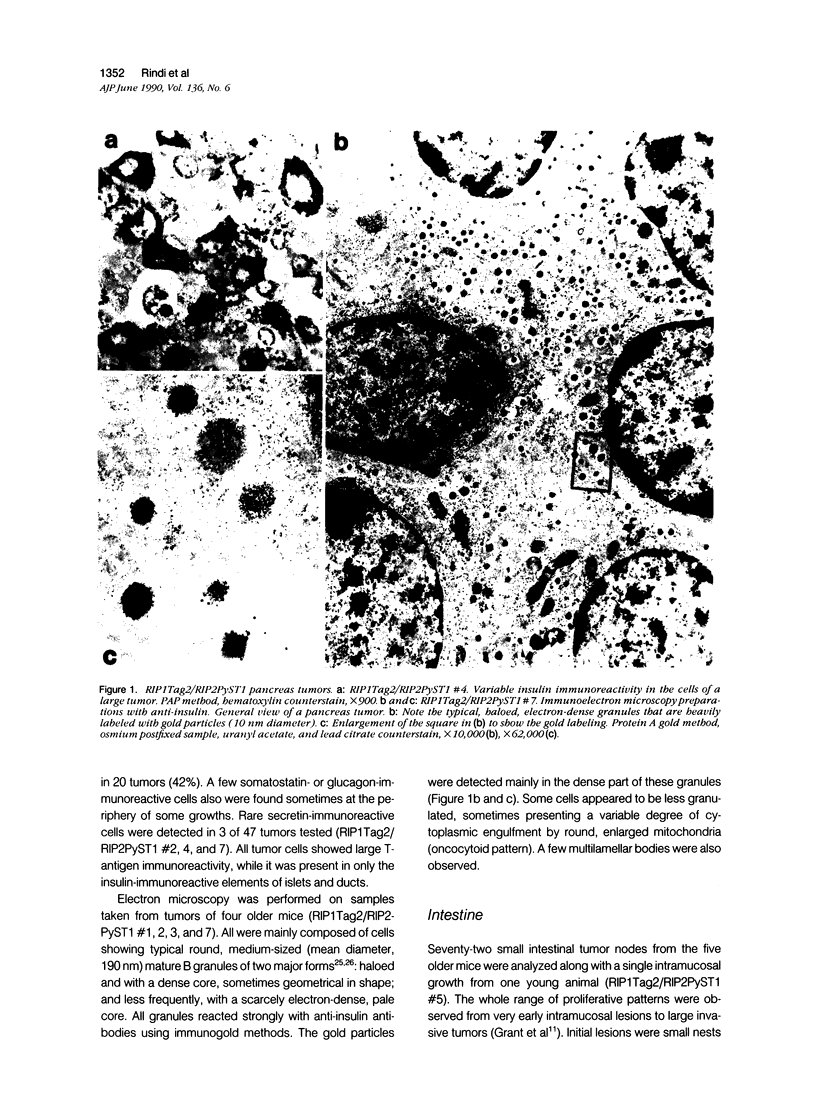
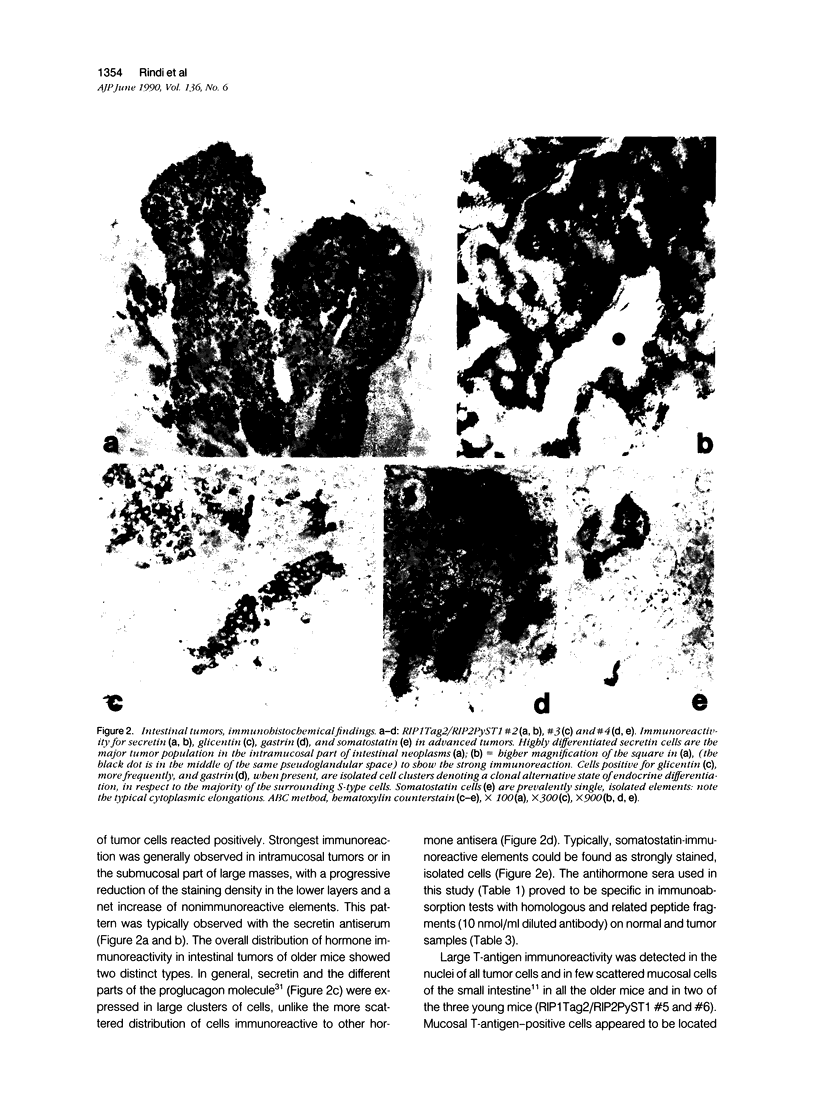
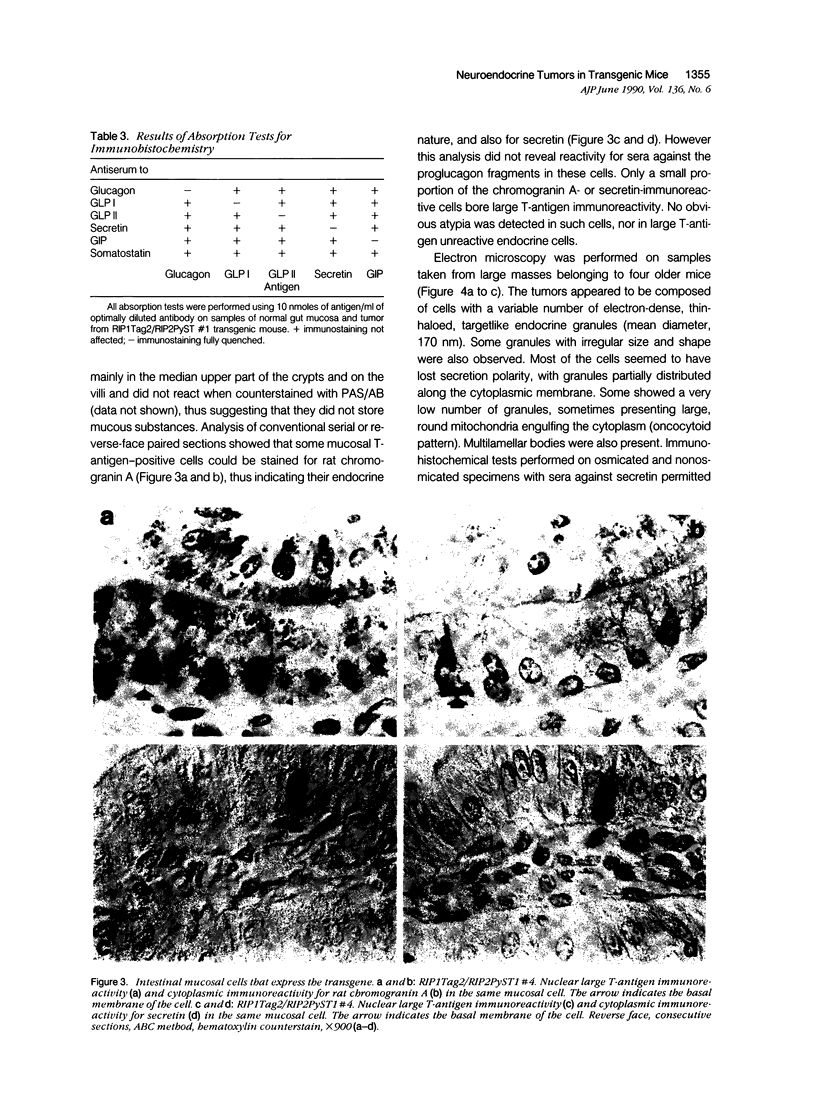
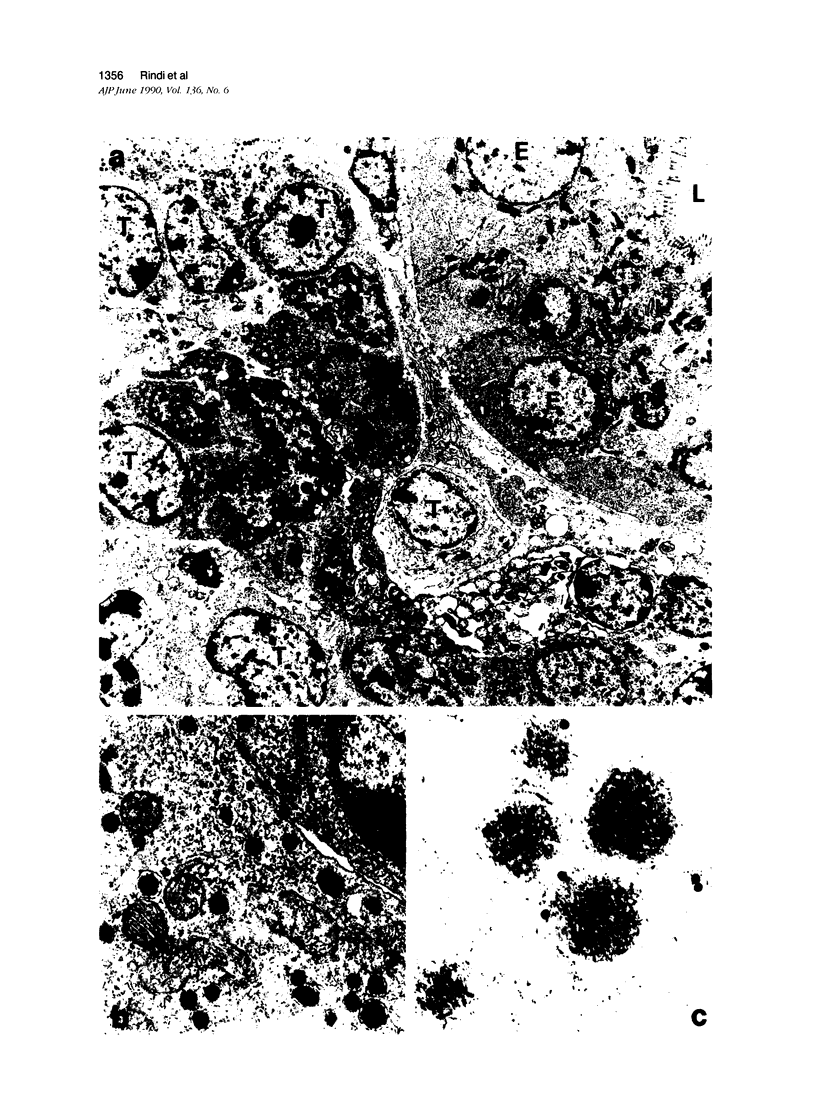
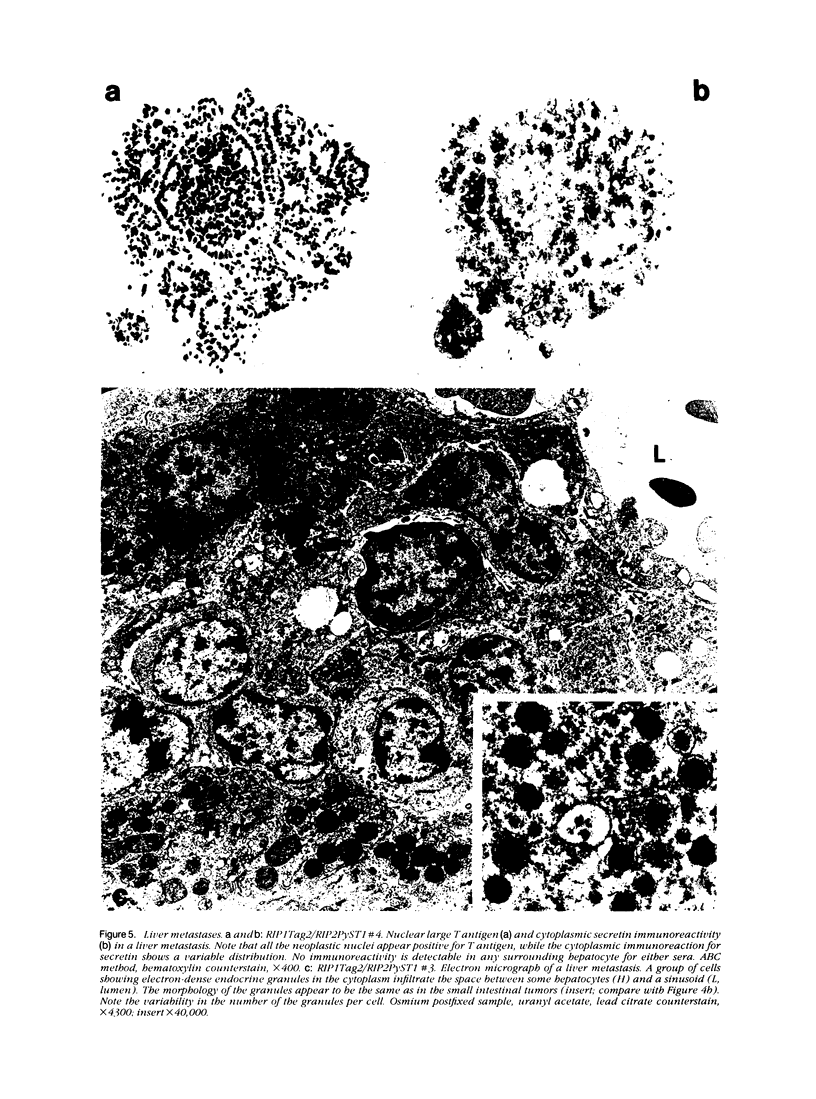
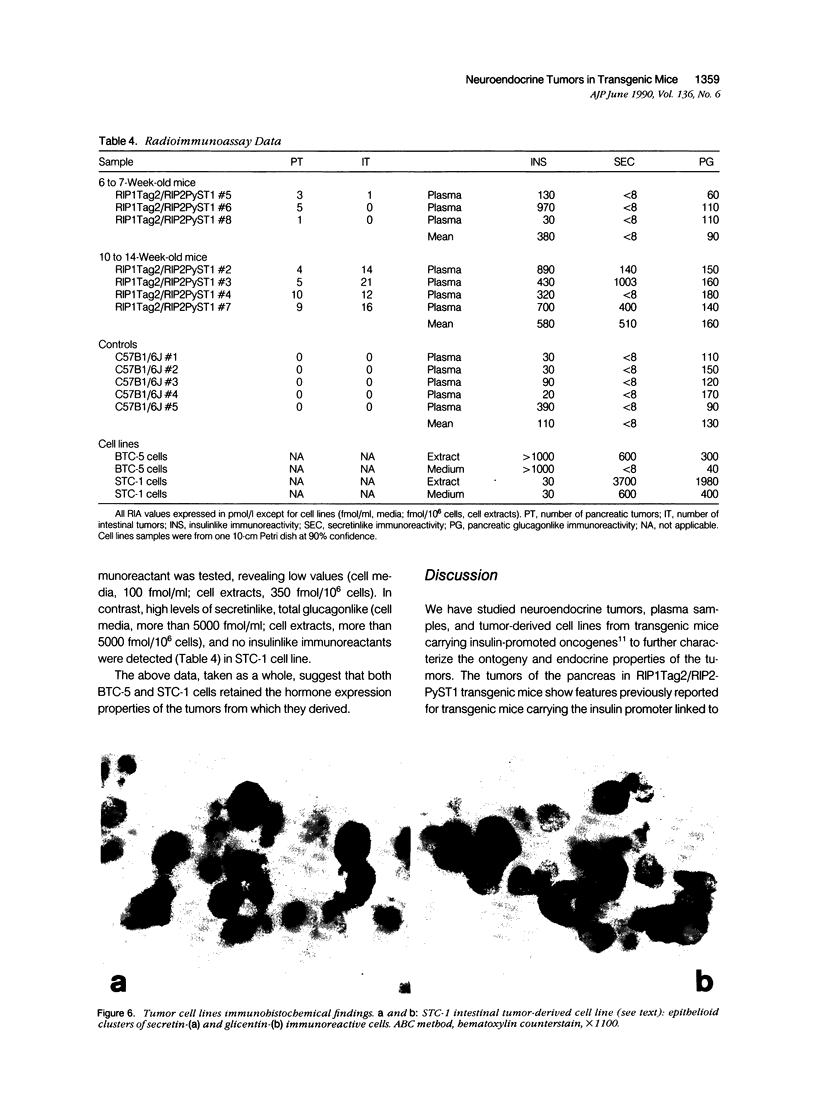
Expression of hormones in endocrine tumors and derived cell lines of transgenic mice carrying insulin-promoted oncogenes has been investigated by histochemical, immunohistochemical, ultrastructural, and radioimmunologic means. Tumors of the pancreas, small intestine, mesentery, and liver were examined. Insulin-immunoreactive cells were prevalent in pancreatic tumors, with a significant subpopulation of pancreatic polypeptide-immunoreactive elements. Conventional ultrastructural and immunogold analysis identified insulin-storing beta granules in pancreatic tumor cells. In contrast, the largest immunoreactive subpopulation of intestinal tumors expressed secretin (53% of total cells), followed by proglucagon-related peptides (15%), glucose-dependent insulinotropic polypeptide (7%), gastrin (7%), pancreatic polypeptide (2%), neurotensin (2%), and somatostatin (1%). No detectable immunoreactivity for either insulin or serotonin was observed. Electron microscopy and immunogold labeling showed that intestinal tumor cells contained secretin-storing S-type granules. Lymph node and liver tumors contained secretin-immunoreactive cells with ultrastructural features similar to those of intestinal tumors. In addition, high levels of circulating insulinlike and secretinlike immunoreactants were detectable. Analogous hormone profiles were identified in tumor cell lines and culture media. Large T-antigen immunoreactivity was detected in all the nuclei of neoplastic cells, as well as in insulin-immunoreactive elements of non-neoplastic islets and pancreatic ducts and in some secretin-immunoreactive cells of small intestinal mucosa. These data indicate that neuroendocrine tumors arise both in beta cell and S-cell subpopulations of transgenic mice.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Bestetti G., Rossi G. L. Islet cell carcinomas in dogs. Virchows Arch A Pathol Anat Histopathol. 1985;405(2):203–214. doi: 10.1007/BF00704372. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974 Dec;141(4):503–519. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Conlon J. M. Proglucagon-derived peptides: nomenclature, biosynthetic relationships and physiological roles. Diabetologia. 1988 Aug;31(8):563–566. doi: 10.1007/BF00264761. [DOI] [PubMed] [Google Scholar]
- Drukker J., Terwindt-Rouwenhorst E. A., Wiertz-Hoessels E. L. Peptide immunoreactivity in developing entero-endocrine cells in endodermal grafts. Histochemistry. 1988;88(3-6):481–484. doi: 10.1007/BF00570312. [DOI] [PubMed] [Google Scholar]
- Efrat S., Teitelman G., Anwar M., Ruggiero D., Hanahan D. Glucagon gene regulatory region directs oncoprotein expression to neurons and pancreatic alpha cells. Neuron. 1988 Sep;1(7):605–613. doi: 10.1016/0896-6273(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Facer P., Bishop A. E., Lloyd R. V., Wilson B. S., Hennessy R. J., Polak J. M. Chromogranin: a newly recognized marker for endocrine cells of the human gastrointestinal tract. Gastroenterology. 1985 Dec;89(6):1366–1373. doi: 10.1016/0016-5085(85)90657-2. [DOI] [PubMed] [Google Scholar]
- Feurle G. E., Helmstaedter V., Tischbirek K., Carraway R., Forssmann W. G., Grube D., Röher H. D. A multihormonal tumor of the pancreas producing neurotensin. Dig Dis Sci. 1981 Dec;26(12):1125–1133. doi: 10.1007/BF01295980. [DOI] [PubMed] [Google Scholar]
- Fiocca R., Rindi G., Capella C., Grimelius L., Polak J. M., Schwartz T. W., Yanaihara N., Solcia E. Glucagon, glicentin, proglucagon, PYY, PP and proPP-icosapeptide immunoreactivities of rectal carcinoid tumors and related non-tumor cells. Regul Pept. 1987 Jan;17(1):9–29. doi: 10.1016/0167-0115(87)90029-2. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie R., Lassmann H., Hagn C., Winkler H. Immunological studies on the distribution of chromogranin A and B in endocrine and nervous tissues. Neuroscience. 1985 Nov;16(3):547–555. doi: 10.1016/0306-4522(85)90191-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Dissecting multistep tumorigenesis in transgenic mice. Annu Rev Genet. 1988;22:479–519. doi: 10.1146/annurev.ge.22.120188.002403. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985 May 9;315(6015):115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- Holm R., Varndell I. M., Power R. F., Bishop A. E., Madsen O. D., Alpert S., Hanahan D., Polak J. M. Ultrastructure and electron immunocytochemistry of insulin-producing B-cell tumors from transgenic mice: comparison with counterpart human tumors. Ultrastruct Pathol. 1988 Sep-Oct;12(5):547–559. doi: 10.3109/01913128809032239. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Heitz P. U. Pancreatic endocrine tumors. Pathol Res Pract. 1988 Apr;183(2):155–168. doi: 10.1016/S0344-0338(88)80043-8. [DOI] [PubMed] [Google Scholar]
- LACY P. E. Electron microscopy of the beta cell of the pancreas. Am J Med. 1961 Dec;31:851–859. doi: 10.1016/0002-9343(61)90024-9. [DOI] [PubMed] [Google Scholar]
- LACY P. E. Electron microscopy of the islets of Langerhans. Diabetes. 1962 Nov-Dec;11:509–513. [PubMed] [Google Scholar]
- Larsson L. I., Sundler F., Alumets J., Håkanson R., Schaffalitzky de Muckadell O. B., Fahrenkrug J. Distribution, ontogeny and ultrastructure of the mammalian secretin cell. Cell Tissue Res. 1977 Jul 15;181(3):361–368. doi: 10.1007/BF00223111. [DOI] [PubMed] [Google Scholar]
- Lloyd R. V., Wilson B. S. Specific endocrine tissue marker defined by a monoclonal antibody. Science. 1983 Nov 11;222(4624):628–630. doi: 10.1126/science.6635661. [DOI] [PubMed] [Google Scholar]
- Madsen O. D., Larsson L. I., Rehfeld J. F., Schwartz T. W., Lernmark A., Labrecque A. D., Steiner D. F. Cloned cell lines from a transplantable islet cell tumor are heterogeneous and express cholecystokinin in addition to islet hormones. J Cell Biol. 1986 Nov;103(5):2025–2034. doi: 10.1083/jcb.103.5.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing A., Chen H. Y., Palmiter R. D., Brinster R. L. Peripheral neuropathies, hepatocellular carcinomas and islet cell adenomas in transgenic mice. Nature. 1985 Aug 1;316(6027):461–463. doi: 10.1038/316461a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki K., Funakoshi A., Nishihara S., Wasada T., Koga A., Ibayashi H. Aberrant insulinoma in the duodenum. Gastroenterology. 1986 May;90(5 Pt 1):1280–1285. doi: 10.1016/0016-5085(86)90397-5. [DOI] [PubMed] [Google Scholar]
- Murphy D., Bishop A., Rindi G., Murphy M. N., Stamp G. W., Hanson J., Polak J. M., Hogan B. Mice transgenic for a vasopressin-SV40 hybrid oncogene develop tumors of the endocrine pancreas and the anterior pituitary. A possible model for human multiple endocrine neoplasia type 1. Am J Pathol. 1987 Dec;129(3):552–566. [PMC free article] [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Chromogranin A: immunohistology reveals its universal occurrence in normal polypeptide hormone producing endocrine glands. Life Sci. 1983 Oct 24;33(17):1657–1663. doi: 10.1016/0024-3205(83)90721-x. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Minna J. D., Weir G. C., Baylin S. B. Clonal analysis of insulin and somatostatin secretion and L-dopa decarboxylase expression by a rat islet cell tumor. Endocrinology. 1983 Mar;112(3):1070–1075. doi: 10.1210/endo-112-3-1070. [DOI] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982 Jun;31(6 Pt 1):538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Pelletier G., Cortot A., Launay J. M., Debons-Guillemain M. C., Nemeth J., Le Charpentier Y., Celerier M., Modigliani R. Serotonin-secreting and insulin-secreting ileal carcinoid tumor and the use of in vitro culture of tumoral cells. Cancer. 1984 Jul 15;54(2):319–322. doi: 10.1002/1097-0142(19840715)54:2<319::aid-cncr2820540224>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Pictet R. L., Rall L. B., Phelps P., Rutter W. J. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science. 1976 Jan 16;191(4223):191–192. doi: 10.1126/science.1108195. [DOI] [PubMed] [Google Scholar]
- Pilato F. P., D'Adda T., Banchini E., Bordi C. Nonrandom expression of polypeptide hormones in pancreatic endocrine tumors. An immunohistochemical study in a case of multiple islet cell neoplasia. Cancer. 1988 May 1;61(9):1815–1820. doi: 10.1002/1097-0142(19880501)61:9<1815::aid-cncr2820610916>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Power R. F., Holm R., Bishop A. E., Varndell I. M., Alpert S., Hanahan D., Polak J. M. Transgenic mouse model: a new approach for the investigation of endocrine pancreatic B-cell growth. Gut. 1987;28 (Suppl):121–129. doi: 10.1136/gut.28.suppl.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi G., Bishop A. E., Murphy D., Solcia E., Hogan B., Polak J. M. A morphological analysis endocrine tumour genesis in pancreas and anterior pituitary of AVP/SV40 transgenic mice. Virchows Arch A Pathol Anat Histopathol. 1988;412(3):255–266. doi: 10.1007/BF00737150. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Immunoreactive secretin in gastrointestinal mucosa of several mammalian species. Gastroenterology. 1978 Sep;75(3):401–404. [PubMed] [Google Scholar]
- Tischler A. S. Gut peptide production by tumour cell lines in culture. Scand J Gastroenterol Suppl. 1983;82:33–43. [PubMed] [Google Scholar]
- Usellini L., Capella C., Frigerio B., Rindi G., Solcia E. Ultrastructural localization of secretin in endocrine cells of the dog duodenum by the immunogold technique. Comparison with ultrastructurally characterized S cells of various mammals. Histochemistry. 1984;80(5):435–441. doi: 10.1007/BF00495431. [DOI] [PubMed] [Google Scholar]
- Varndell I. M., Tapia F. J., Probert L., Buchan A. M., Gu J., De Mey J., Bloom S. R., Polak J. M. Immunogold staining procedure for the localisation of regulatory peptides. Peptides. 1982 May-Jun;3(3):259–272. doi: 10.1016/0196-9781(82)90086-9. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]