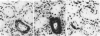Abstract
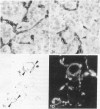
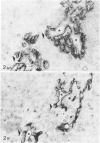
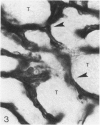
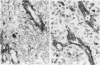
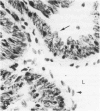
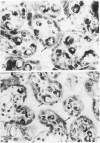
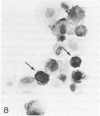
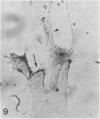
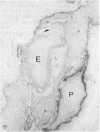
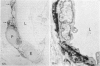
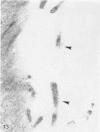
In the course of immunohistochemical characterization of murine monoclonal antibodies recognizing the human high molecular weight-melanoma associated antigen (HMW-MAA), a striking reactivity with blood vessels in the tumor stroma was noted. Immunocytochemical analysis by light and electronmicroscopy of a panel of tissues and cell lines showed that the staining of microvessels by anti-HMW-MAA monoclonal antibodies was restricted to pericytes. Correspondingly, anti-HMW-MAA monoclonal antibodies were found to react with cultured pericytes from human brain, but not with endothelial cells in serologic assays, and to immunoprecipitate from biosynthetically labeled pericytes an antigen with the characteristic structural profile of HMW-MAA. At the subcellular level, the expression of HMW-MAA in cultured pericytes was mainly restricted to microspikes that are localized in clusters on the cellular membrane. Staining by anti-HMW-MAA monoclonal antibodies of pericytes was not only found in the tumor stroma, but also in other lesions associated with angiogenesis, such as granulation tissue of wound healing and synovitis. In these lesions, microvascular staining for another marker of pericytes, ie, alpha-smooth muscle actin, also was observed. These results suggest that, in conditions associated with vascular proliferation, 1) pericytes acquire HMW-MAA and 2) the number of pericytes may be increased as compared with normal tissues.
Full text
PDF

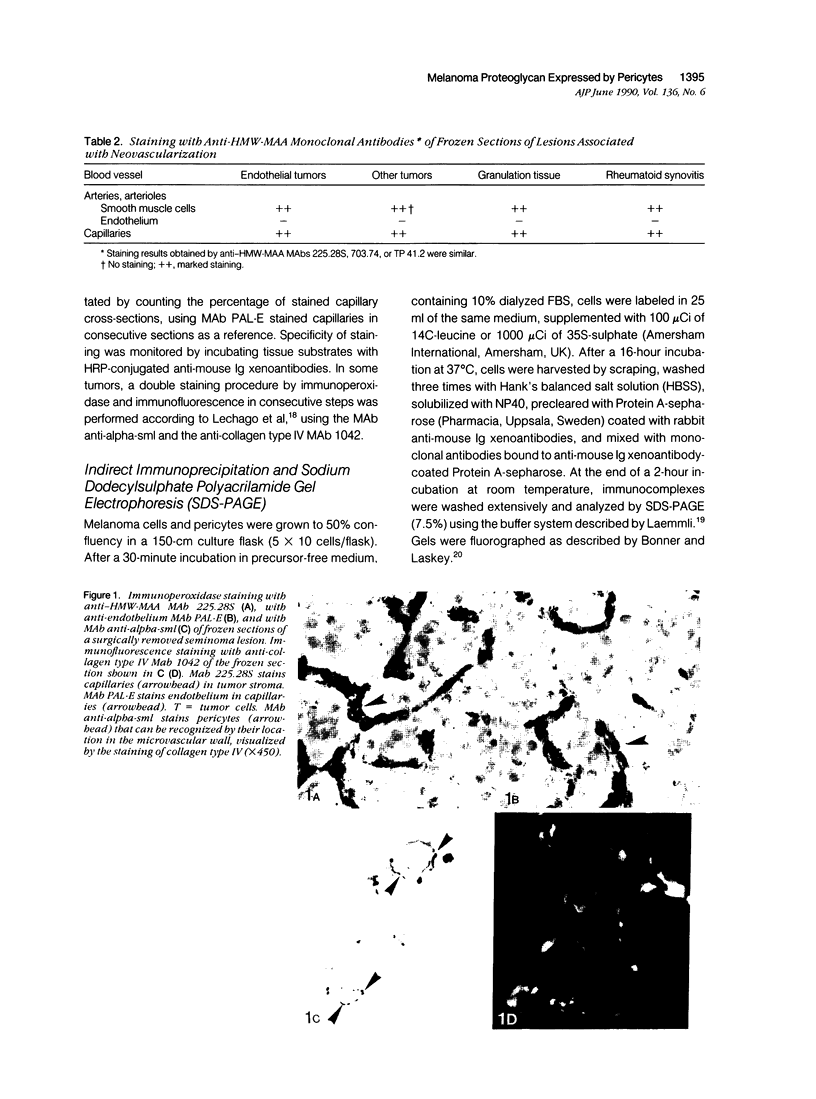
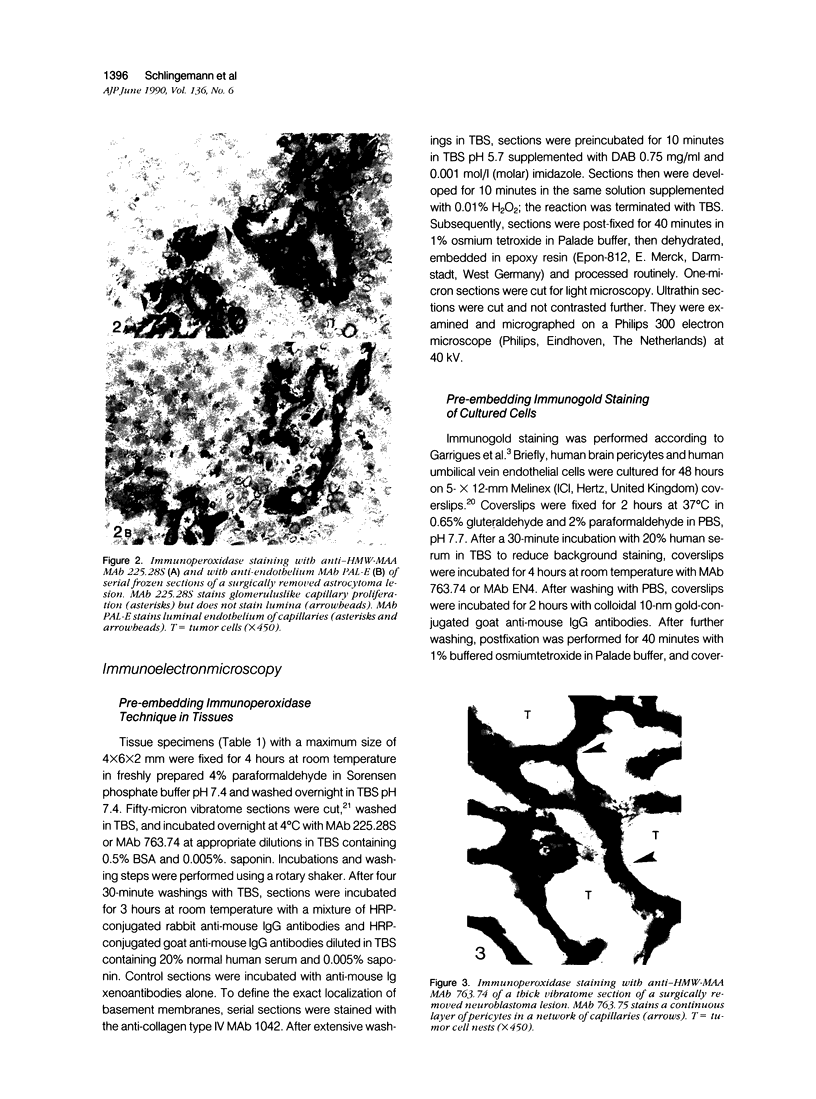



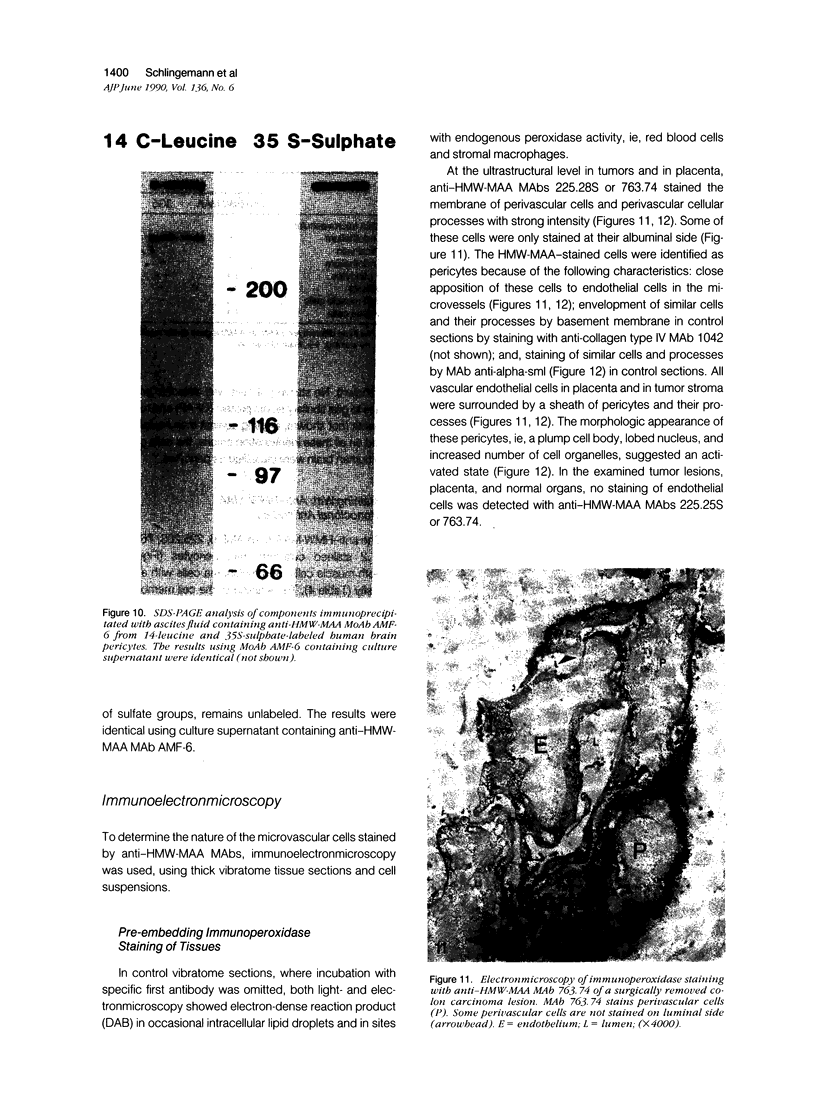

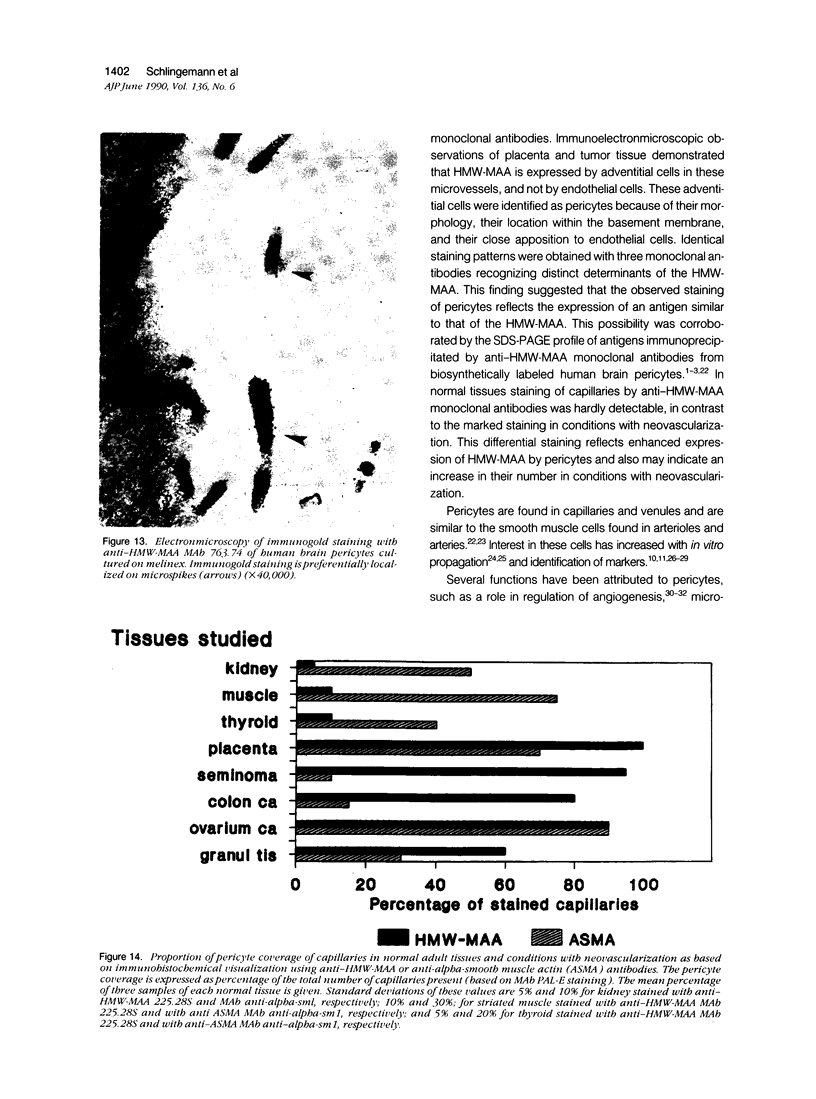



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. Filopodia of spreading 3T3 cells. Do they have a substrate-exploring function? J Cell Biol. 1976 May;69(2):275–286. doi: 10.1083/jcb.69.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumol T. F., Walker L. E., Reisfeld R. A. Biosynthetic studies of proteoglycans in human melanoma cells with a monoclonal antibody to a core glycoprotein of chondroitin sulfate proteoglycans. J Biol Chem. 1984 Oct 25;259(20):12733–12741. [PubMed] [Google Scholar]
- Carson M. P., Haudenschild C. C. Microvascular endothelium and pericytes: high yield, low passage cultures. In Vitro Cell Dev Biol. 1986 Jun;22(6):344–354. doi: 10.1007/BF02623409. [DOI] [PubMed] [Google Scholar]
- Cavallo T., Sade R., Folkman J., Cotran R. S. Ultrastructural autoradiographic studies of the early vasoproliferative response in tumor angiogenesis. Am J Pathol. 1973 Mar;70(3):345–362. [PMC free article] [PubMed] [Google Scholar]
- Crocker D. J., Murad T. M., Geer J. C. Role of the pericyte in wound healing. An ultrastructural study. Exp Mol Pathol. 1970 Aug;13(1):51–65. doi: 10.1016/0014-4800(70)90084-5. [DOI] [PubMed] [Google Scholar]
- Davison P. M., Bensch K., Karasek M. A. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Invest Dermatol. 1980 Oct;75(4):316–321. doi: 10.1111/1523-1747.ep12530941. [DOI] [PubMed] [Google Scholar]
- Denekamp J. Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br J Cancer. 1982 Jan;45(1):136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Furcht L. T. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986 Nov;55(5):505–509. [PubMed] [Google Scholar]
- Garin-Chesa P., Beresford H. R., Carrato-Mena A., Oettgen H. F., Old L. J., Melamed M. R., Rettig W. J. Cell surface molecules of human melanoma. Immunohistochemical analysis of the gp57, GD3, and mel-CSPG antigenic systems. Am J Pathol. 1989 Feb;134(2):295–303. [PMC free article] [PubMed] [Google Scholar]
- Garrigues H. J., Lark M. W., Lara S., Hellström I., Hellström K. E., Wight T. N. The melanoma proteoglycan: restricted expression on microspikes, a specific microdomain of the cell surface. J Cell Biol. 1986 Nov;103(5):1699–1710. doi: 10.1083/jcb.103.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlowski L. E., Jain R. K. Microvascular permeability of normal and neoplastic tissues. Microvasc Res. 1986 May;31(3):288–305. doi: 10.1016/0026-2862(86)90018-x. [DOI] [PubMed] [Google Scholar]
- Hagemeier H. H., Vollmer E., Goerdt S., Schulze-Osthoff K., Sorg C. A monoclonal antibody reacting with endothelial cells of budding vessels in tumors and inflammatory tissues, and non-reactive with normal adult tissues. Int J Cancer. 1986 Oct 15;38(4):481–488. doi: 10.1002/ijc.2910380405. [DOI] [PubMed] [Google Scholar]
- Harper J. R., Reisfeld R. A. Inhibition of anchorage-independent growth of human melanoma cells by a monoclonal antibody to a chondroitin sulfate proteoglycan. J Natl Cancer Inst. 1983 Aug;71(2):259–263. [PubMed] [Google Scholar]
- Herlyn M., Koprowski H. Melanoma antigens: immunological and biological characterization and clinical significance. Annu Rev Immunol. 1988;6:283–308. doi: 10.1146/annurev.iy.06.040188.001435. [DOI] [PubMed] [Google Scholar]
- Herman I. M., D'Amore P. A. Microvascular pericytes contain muscle and nonmuscle actins. J Cell Biol. 1985 Jul;101(1):43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser L. S., Miller F. N. Differential macromolecular leakage from the vasculature of tumors. Cancer. 1986 Feb 1;57(3):461–464. doi: 10.1002/1097-0142(19860201)57:3<461::aid-cncr2820570310>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Ho K. L. Ultrastructure of cerebellar capillary hemangioblastoma. IV. Pericytes and their relationship to endothelial cells. Acta Neuropathol. 1985;67(3-4):254–264. doi: 10.1007/BF00687810. [DOI] [PubMed] [Google Scholar]
- Hobson B., Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984 Apr;49(4):405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Biol. 1985 May;100(5):1379–1386. doi: 10.1083/jcb.100.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J Cell Biol. 1985 May;100(5):1387–1395. doi: 10.1083/jcb.100.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUWABARA T., COGAN D. G. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963 Apr;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- Krause D., Vatter B., Dermietzel R. Immunochemical and immunocytochemical characterization of a novel monoclonal antibody recognizing a 140 kDa protein in cerebral pericytes of the rat. Cell Tissue Res. 1988 Jun;252(3):543–555. doi: 10.1007/BF00216641. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lechago J., Sun N. C., Weinstein W. M. Simultaneous visualization of two antigens in the same tissue section by combining immunoperoxidase with immunofluorescence techniques. J Histochem Cytochem. 1979 Sep;27(9):1221–1225. doi: 10.1177/27.9.113453. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Albino A., Houghton A. Analysis of hybridoma-exchange antibodies. Hybridoma. 1982;1(4):461–463. doi: 10.1089/hyb.1.1982.1.461. [DOI] [PubMed] [Google Scholar]
- Nakane P K, Pierce G B., Jr Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem. 1966 Dec;14(12):929–931. doi: 10.1177/14.12.929. [DOI] [PubMed] [Google Scholar]
- Orlidge A., D'Amore P. A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J Cell Biol. 1987 Sep;105(3):1455–1462. doi: 10.1083/jcb.105.3.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold H. S., van den Berg-Blok A. Factors influencing the neovascularization of experimental tumours. Biorheology. 1984;21(4):493–501. doi: 10.3233/bir-1984-21408. [DOI] [PubMed] [Google Scholar]
- Ruiter D. J., Schlingemann R. O., Rietveld F. J., de Waal R. M. Monoclonal antibody-defined human endothelial antigens as vascular markers. J Invest Dermatol. 1989 Aug;93(2 Suppl):25S–32S. doi: 10.1111/1523-1747.ep12580902. [DOI] [PubMed] [Google Scholar]
- SCHOEFL G. I. STUDIES ON INFLAMMATION. III. GROWING CAPILLARIES: THEIR STRUCTURE AND PERMEABILITY. Virchows Arch Pathol Anat Physiol Klin Med. 1963 Nov 8;337:97–141. [PubMed] [Google Scholar]
- Schlingemann R. O., Dingjan G. M., Emeis J. J., Blok J., Warnaar S. O., Ruiter D. J. Monoclonal antibody PAL-E specific for endothelium. Lab Invest. 1985 Jan;52(1):71–76. [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987 Oct;1(4):272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L. The isolation and culture of endothelial cells and pericytes from the bovine retinal microvasculature: a comparative study with large vessel vascular cells. Microvasc Res. 1986 Jul;32(1):21–38. doi: 10.1016/0026-2862(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Scopsi L., Larsson L. I. Increased sensitivity in peroxidase immunocytochemistry. A comparative study of a number of peroxidase visualization methods employing a model system. Histochemistry. 1986;84(3):221–230. doi: 10.1007/BF00495786. [DOI] [PubMed] [Google Scholar]
- Sholley M. M., Cavallo T., Cotran R. S. Endothelial proliferation in inflammation. I. Autoradiographic studies following thermal injury to the skin of normal rats. Am J Pathol. 1977 Nov;89(2):277–296. [PMC free article] [PubMed] [Google Scholar]
- Sims D. E. The pericyte--a review. Tissue Cell. 1986;18(2):153–174. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F., Hayashi S. The proliferation of capillary endothelial cells. Cancer Res. 1972 Jan;32(1):77–82. [PubMed] [Google Scholar]
- Verhoeven D., Buyssens N. Desmin-positive stellate cells associated with angiogenesis in a tumour and non-tumour system. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54(5):263–272. doi: 10.1007/BF02899222. [DOI] [PubMed] [Google Scholar]
- Vinters H. V., Reave S., Costello P., Girvin J. P., Moore S. A. Isolation and culture of cells derived from human cerebral microvessels. Cell Tissue Res. 1987 Sep;249(3):657–667. doi: 10.1007/BF00217338. [DOI] [PubMed] [Google Scholar]
- Weber K., Braun-Falco O. Ultrastructure of blood vessels in human granulation tissue. Arch Dermatol Forsch. 1973;248(1):29–44. doi: 10.1007/BF00594710. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Imai K., Natali P. G., Ferrone S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int J Cancer. 1981 Sep 15;28(3):293–300. doi: 10.1002/ijc.2910280307. [DOI] [PubMed] [Google Scholar]
- Ziai M. R., Imberti L., Nicotra M. R., Badaracco G., Segatto O., Natali P. G., Ferrone S. Analysis with monoclonal antibodies of the molecular and cellular heterogeneity of human high molecular weight melanoma associated antigen. Cancer Res. 1987 May 1;47(9):2474–2480. [PubMed] [Google Scholar]
- de Vries J. E., Keizer G. D., te Velde A. A., Voordouw A., Ruiter D., Rümke P., Spits H., Figdor C. G. Characterization of melanoma-associated surface antigens involved in the adhesion and motility of human melanoma cells. Int J Cancer. 1986 Oct 15;38(4):465–473. doi: 10.1002/ijc.2910380403. [DOI] [PubMed] [Google Scholar]
- van Duinen S. G., Mauw B. J., de Graaff-Reitsma C. B., Ruiter D. J. Methods in laboratory investigation. Immunoelectron microscopic methods for demonstration of antigens on normal human melanocytes and other epidermal cells. Lab Invest. 1984 Jun;50(6):733–741. [PubMed] [Google Scholar]
- van Hinsbergh V. W., Havekes L., Emeis J. J., van Corven E., Scheffer M. Low density lipoprotein metabolism by endothelial cells from human umbilical cord arteries and veins. Arteriosclerosis. 1983 Nov-Dec;3(6):547–559. doi: 10.1161/01.atv.3.6.547. [DOI] [PubMed] [Google Scholar]