Abstract
To examine the effects of recombinant growth factors in vivo, impaired wound healing was studied in genetically diabetic C57BL/KsJ-db/db mice. Large full-thickness skin wounds made on the backs of these mice exhibited significant delays in the entry of inflammatory cells into the wound, the formation of granulation tissue, and in wound closure when compared to their nondiabetic littermates. Recombinant human platelet-derived growth factor (rPDGF-BB, 1 or 10 micrograms), recombinant human basic fibroblast growth factor (rbFGF, 1 micrograms), or combinations of both were applied topically to the wounds for 5 to 14 days after wounding. Diabetic mouse wounds treated with rPDGF-BB or rbFGF had many more fibroblasts and capillaries in the wound bed at 10 and 21 days than did wounds treated with the vehicle alone. The animals treated with growth factors also had significantly greater wound closure at 21 days than those treated with the vehicle. Combinations of rPDGF-BB and rbFGF improved all parameters of healing but not to a greater extent than either growth factor alone. The effectiveness of rPDGF-BB and rbFGF suggest that recombinant growth factors may be useful in the treatment of patients with deficient wound repair.
Full text
PDF


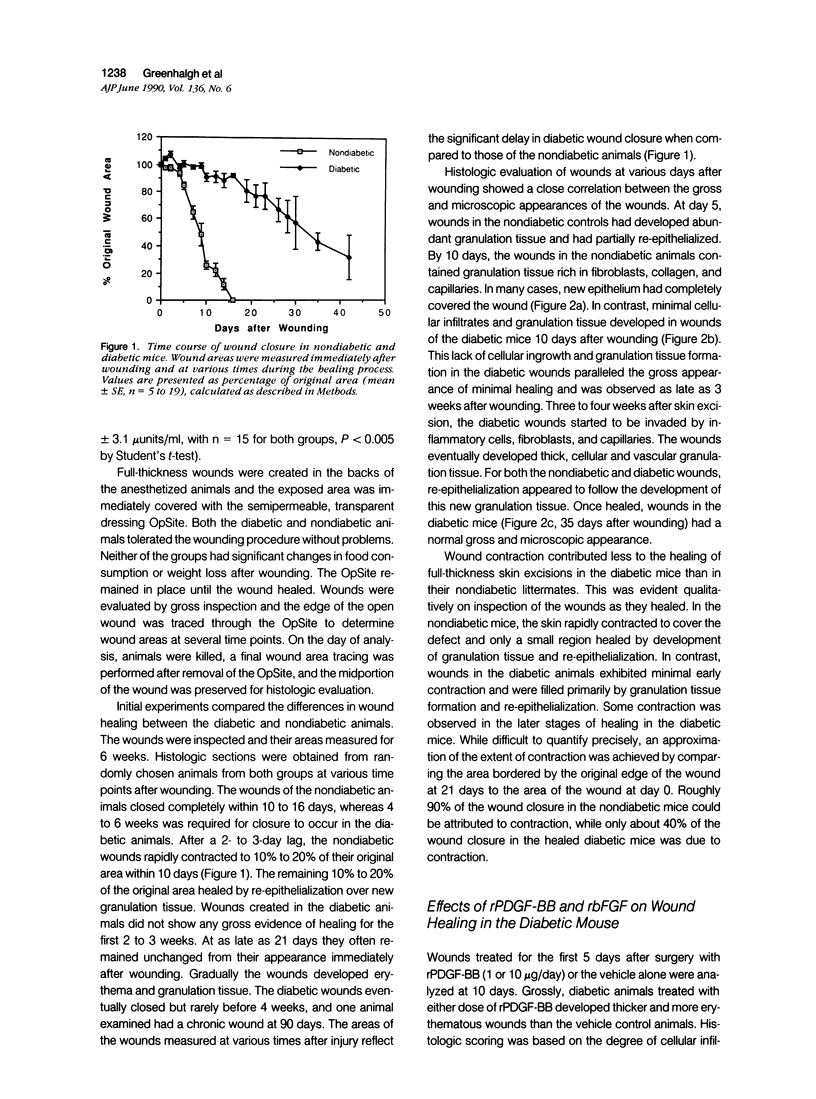
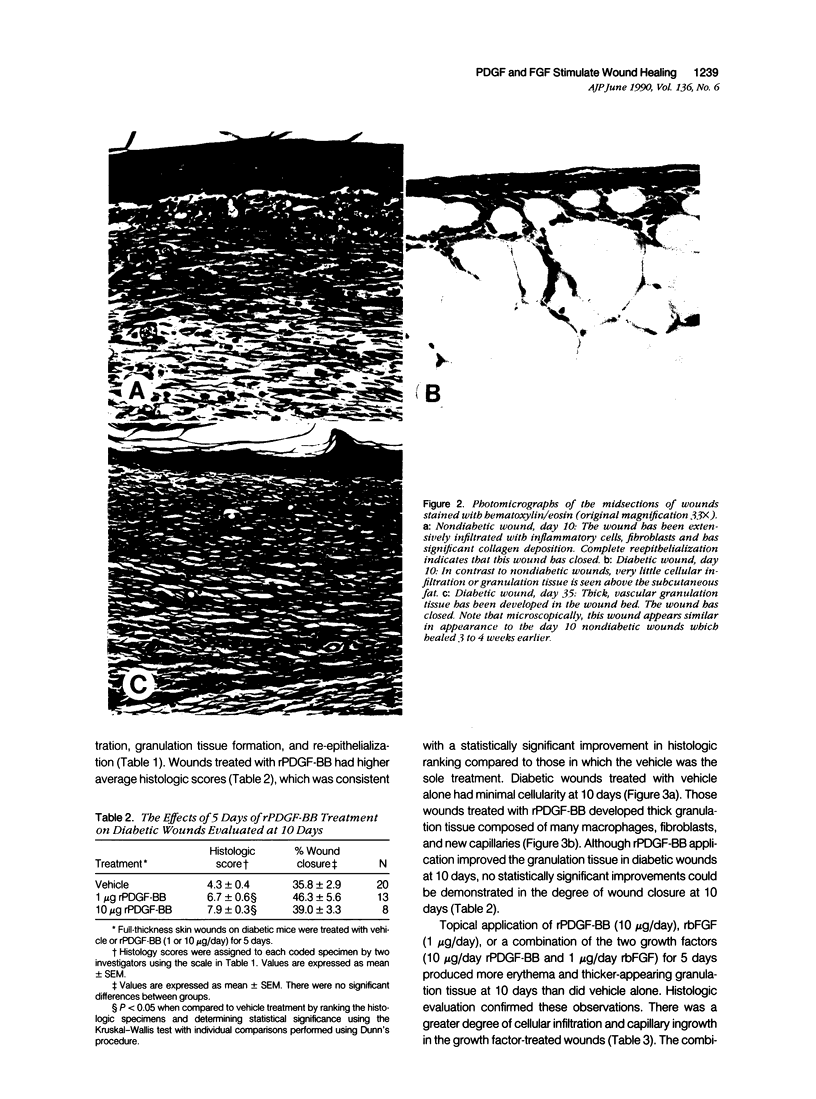
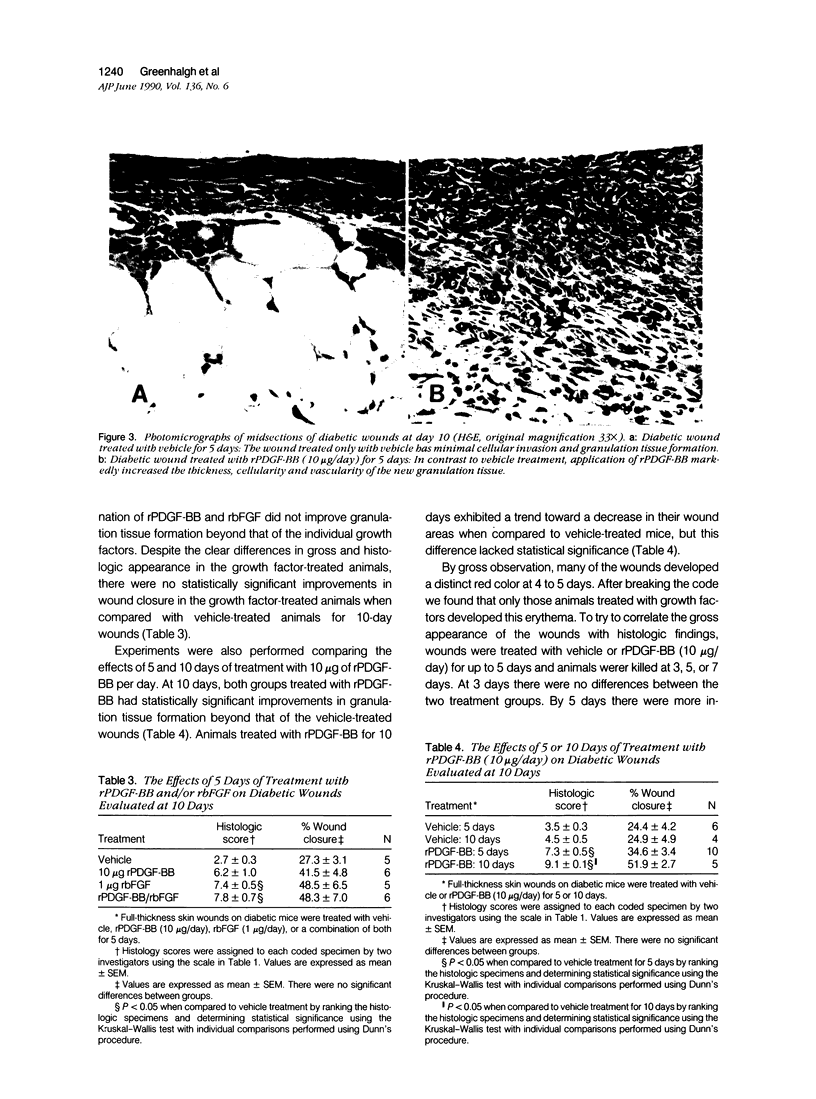



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer E. A., Cooper T. W., Huang J. S., Altman J., Deuel T. F. Stimulation of in vitro human skin collagenase expression by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4132–4136. doi: 10.1073/pnas.82.12.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Brown G. L., Curtsinger L., 3rd, Brightwell J. R., Ackerman D. M., Tobin G. R., Polk H. C., Jr, George-Nascimento C., Valenzuela P., Schultz G. S. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986 May 1;163(5):1319–1324. doi: 10.1084/jem.163.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J., 3rd, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989 Jul 13;321(2):76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- Buntrock P., Jentzsch K. D., Heder G. Stimulation of wound healing, using brain extract with fibroblast growth factor (FGF) activity. I. Quantitative and biochemical studies into formation of granulation tissue. Exp Pathol. 1982;21(1):46–53. doi: 10.1016/s0232-1513(82)80051-0. [DOI] [PubMed] [Google Scholar]
- Böhlen P., Baird A., Esch F., Ling N., Gospodarowicz D. Isolation and partial molecular characterization of pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5364–5368. doi: 10.1073/pnas.81.17.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua C. C., Geiman D. E., Keller G. H., Ladda R. L. Induction of collagenase secretion in human fibroblast cultures by growth promoting factors. J Biol Chem. 1985 May 10;260(9):5213–5216. [PubMed] [Google Scholar]
- Coleman D. L. Diabetes-obesity syndromes in mice. Diabetes. 1982;31(Suppl 1 Pt 2):1–6. doi: 10.2337/diab.31.1.s1. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978 Mar;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Davidson J. M., Klagsbrun M., Hill K. E., Buckley A., Sullivan R., Brewer P. S., Woodward S. C. Accelerated wound repair, cell proliferation, and collagen accumulation are produced by a cartilage-derived growth factor. J Cell Biol. 1985 Apr;100(4):1219–1227. doi: 10.1083/jcb.100.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray-Sachs M., Dardenne M., Sai P., Savino W., Quiniou M. C., Boillot D., Gepts W., Assan R. Anti-islet immunity and thymic dysfunction in the mutant diabetic C57BL/KsJ db/db mouse. Diabetes. 1983 Nov;32(11):1048–1054. doi: 10.2337/diab.32.11.1048. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourtanier A. Y., Courty J., Muller E., Courtois Y., Prunieras M., Barritault D. Eye-derived growth factor isolated from bovine retina and used for epidermal wound healing in vivo. J Invest Dermatol. 1986 Jul;87(1):76–80. doi: 10.1111/1523-1747.ep12523578. [DOI] [PubMed] [Google Scholar]
- Goodson W. H., 3rd, Hung T. K. Studies of wound healing in experimental diabetes mellitus. J Surg Res. 1977 Mar;22(3):221–227. doi: 10.1016/0022-4804(77)90137-8. [DOI] [PubMed] [Google Scholar]
- Goodson W. H., 3rd, Hunt T. K. Deficient collagen formation by obese mice in a standard wound model. Am J Surg. 1979 Nov;138(5):692–694. doi: 10.1016/0002-9610(79)90350-7. [DOI] [PubMed] [Google Scholar]
- Goodson W. H., 3rd, Hunt T. K. Wound collagen accumulation in obese hyperglycemic mice. Diabetes. 1986 Apr;35(4):491–495. doi: 10.2337/diab.35.4.491. [DOI] [PubMed] [Google Scholar]
- Goodson W. H., 3rd, Hunt T. K. Wound healing and the diabetic patient. Surg Gynecol Obstet. 1979 Oct;149(4):600–608. [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J. S. Mitogenic effect of fibroblast growth factor on early passage cultures of human and murine fibroblasts. J Cell Biol. 1975 Aug;66(2):451–457. doi: 10.1083/jcb.66.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Neufeld G., Schweigerer L. Molecular and biological characterization of fibroblast growth factor, an angiogenic factor which also controls the proliferation and differentiation of mesoderm and neuroectoderm derived cells. Cell Differ. 1986 Jul;19(1):1–17. doi: 10.1016/0045-6039(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Gottrup F., Andreassen T. T. Healing of incisional wounds in stomach and duodenum: the influence of experimental diabetes. J Surg Res. 1981 Jul;31(1):61–68. doi: 10.1016/0022-4804(81)90030-5. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Martin G. R., Pencev D., Sodek J., Harvey A. K. Stimulation of granulation tissue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985 Dec;76(6):2323–2329. doi: 10.1172/JCI112243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar K. A., Hajjar D. P., Silverstein R. L., Nachman R. L. Tumor necrosis factor-mediated release of platelet-derived growth factor from cultured endothelial cells. J Exp Med. 1987 Jul 1;166(1):235–245. doi: 10.1084/jem.166.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Irvin G. L., 3rd, Robinson D. S., Hubbard S. Operative risks in patients with colorectal cancer. Am Surg. 1985 Jul;51(7):418–422. [PubMed] [Google Scholar]
- Kelly J. D., Raines E. W., Ross R., Murray M. J. The B chain of PDGF alone is sufficient for mitogenesis. EMBO J. 1985 Dec 16;4(13A):3399–3405. doi: 10.1002/j.1460-2075.1985.tb04096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Ciresi K. F., Fiegel V. D., Austin L. L., Butler E. L. Classification and treatment of chronic nonhealing wounds. Successful treatment with autologous platelet-derived wound healing factors (PDWHF). Ann Surg. 1986 Sep;204(3):322–330. doi: 10.1097/00000658-198609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Thakral K. K., Goodson W. H., 3rd Role of platelets and fibrin in the healing sequence: an in vivo study of angiogenesis and collagen synthesis. Ann Surg. 1982 Oct;196(4):379–388. doi: 10.1097/00000658-198210000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence W. T., Norton J. A., Sporn M. B., Gorschboth C., Grotendorst G. R. The reversal of an Adriamycin induced healing impairment with chemoattractants and growth factors. Ann Surg. 1986 Feb;203(2):142–147. doi: 10.1097/00000658-198602000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitzel K., Cano C., Marks J. G., Jr, Lipton A. Growth factors and wound healing in the hamster. J Dermatol Surg Oncol. 1985 Jun;11(6):617–622. doi: 10.1111/j.1524-4725.1985.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Lobb R., Sasse J., Sullivan R., Shing Y., D'Amore P., Jacobs J., Klagsbrun M. Purification and characterization of heparin-binding endothelial cell growth factors. J Biol Chem. 1986 Feb 5;261(4):1924–1928. [PubMed] [Google Scholar]
- Lynch S. E., Nixon J. C., Colvin R. B., Antoniades H. N. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7696–7700. doi: 10.1073/pnas.84.21.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee G. S., Davidson J. M., Buckley A., Sommer A., Woodward S. C., Aquino A. M., Barbour R., Demetriou A. A. Recombinant basic fibroblast growth factor accelerates wound healing. J Surg Res. 1988 Jul;45(1):145–153. doi: 10.1016/0022-4804(88)90034-0. [DOI] [PubMed] [Google Scholar]
- Mordes J. P., Rossini A. A. Animal models of diabetes. Am J Med. 1981 Feb;70(2):353–360. doi: 10.1016/0002-9343(81)90772-5. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Narayanan A. S., Page R. C. Biosynthesis and regulation of type V collagen in diploid human fibroblasts. J Biol Chem. 1983 Oct 10;258(19):11694–11699. [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Lingelbach J., Masakowski V. R., Gramates P., Deuel T. F. Transforming growth factor beta reverses the glucocorticoid-induced wound-healing deficit in rats: possible regulation in macrophages by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2229–2233. doi: 10.1073/pnas.86.7.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Mustoe T. A., Senior R. M., Reed J., Griffin G. L., Thomason A., Deuel T. F. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J Exp Med. 1988 Mar 1;167(3):974–987. doi: 10.1084/jem.167.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole G. V., Jr Mechanical factors in abdominal wound closure: the prevention of fascial dehiscence. Surgery. 1985 Jun;97(6):631–640. [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Wakefield L. M., Roche N. S., Stern D. F., Sporn M. B. Type beta transforming growth factor: a bifunctional regulator of cellular growth. Proc Natl Acad Sci U S A. 1985 Jan;82(1):119–123. doi: 10.1073/pnas.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage K., Siebert E., Swann D. The effect of platelet-derived growth factor on cell division and glycosaminoglycan synthesis by human skin and scar fibroblasts. J Invest Dermatol. 1987 Jul;89(1):93–99. doi: 10.1111/1523-1747.ep12580438. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Schrock T. R., Deveney C. W., Dunphy J. E. Factor contributing to leakage of colonic anastomoses. Ann Surg. 1973 May;177(5):513–518. doi: 10.1097/00000658-197305000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Seifter E., Rettura G., Padawer J., Stratford F., Kambosos D., Levenson S. M. Impaired wound healing in streptozotocin diabetes. Prevention by supplemental vitamin A. Ann Surg. 1981 Jul;194(1):42–50. doi: 10.1097/00000658-198107000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Huang S. S., Griffin G. L., Huang J. S. Brain-derived growth factor is a chemoattractant for fibroblasts and astroglial cells. Biochem Biophys Res Commun. 1986 Nov 26;141(1):67–72. doi: 10.1016/s0006-291x(86)80335-7. [DOI] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprugel K. H., McPherson J. M., Clowes A. W., Ross R. Effects of growth factors in vivo. I. Cell ingrowth into porous subcutaneous chambers. Am J Pathol. 1987 Dec;129(3):601–613. [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., DiFlorio R., Lyall R. M., Hic S., Friesel R., Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol. 1985 Dec;101(6):2330–2334. doi: 10.1083/jcb.101.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woost P. G., Brightwell J., Eiferman R. A., Schultz G. S. Effect of growth factors with dexamethasone on healing of rabbit corneal stromal incisions. Exp Eye Res. 1985 Jan;40(1):47–60. doi: 10.1016/0014-4835(85)90107-1. [DOI] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Marsh M., Mai Y. W., Spaliviero J., Delbridge L., Reeve T., Turtle J. R. Effects of experimental diabetes, uremia, and malnutrition on wound healing. Diabetes. 1987 Mar;36(3):295–299. doi: 10.2337/diab.36.3.295. [DOI] [PubMed] [Google Scholar]