Abstract
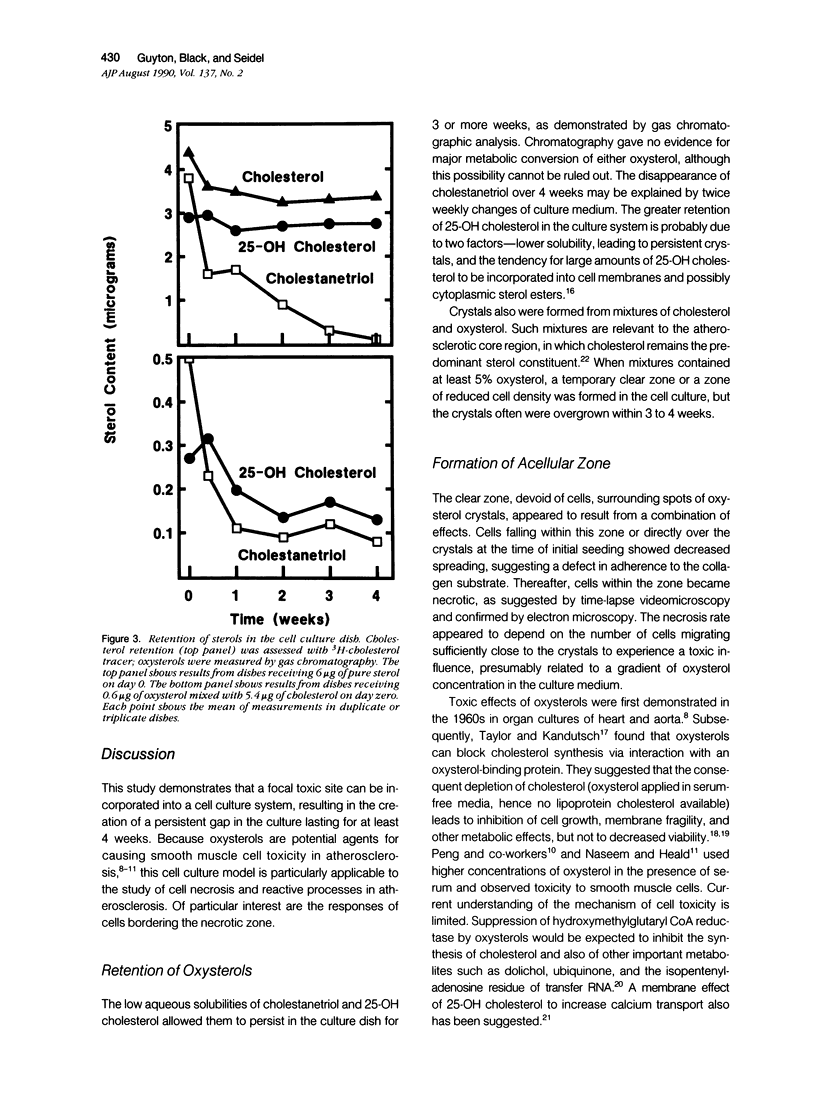
Cell necrosis and reactive cellular processes in and near the atherosclerotic core region might result from short-range interactions with toxic lipids. To model these interactions in cell culture, focal crystalline deposits of cholestane-3 beta,5 alpha,6 beta-triol, 25-OH cholesterol, and cholesterol were overlaid by a collagen gel, on which canine aortic smooth muscle cells were seeded. Oxysterols, but not cholesterol, caused focally decreased plating efficiency and cell death, leading to the formation of a persistent circular gap in the cell culture. Cholestanetriol was largely removed from the culture dishes over 3 to 4 weeks, whereas cholesterol and 25-OH cholesterol were largely retained. Smooth muscle cells were motile even in proximity to oxysterol crystals, with occasional suicidal migration toward the crystals. Chemoattraction, however, could not be demonstrated. Despite toxicity, cholestanetriol did not appear to alter the fraction of cells exhibiting 3H-thymidine uptake, even in areas close to the crystals. Thus, oxysterols may be toxic to some cells, without causing major impairment of the migration and proliferation of nearby cells. This would allow the simultaneous occurrence of cell death and proliferation evident in atherosclerosis.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bocan T. M., Guyton J. R. Human aortic fibrolipid lesions. Progenitor lesions for fibrous plaques, exhibiting early formation of the cholesterol-rich core. Am J Pathol. 1985 Aug;120(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Bocan T. M., Schifani T. A., Guyton J. R. Ultrastructure of the human aortic fibrolipid lesion. Formation of the atherosclerotic lipid-rich core. Am J Pathol. 1986 Jun;123(3):413–424. [PMC free article] [PubMed] [Google Scholar]
- Brown B. G., Fry D. L. The fate and fibrogenic potential of subintimal implants of crystalline lipid in the canine aorta. Quantitative histological and autoradiographic studies. Circ Res. 1978 Aug;43(2):261–273. doi: 10.1161/01.res.43.2.261. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Cholesterol ester formation in cultured human fibroblasts. Stimulation by oxygenated sterols. J Biol Chem. 1975 May 25;250(10):4025–4027. [PubMed] [Google Scholar]
- Byer J. A., Easton J. D. Therapy of ischemic cerebrovascular disease. Ann Intern Med. 1980 Nov;93(5):742–756. doi: 10.7326/0003-4819-93-5-742. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Stoker M. G. Conditions determining initiation of DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1970 May;66(1):204–210. doi: 10.1073/pnas.66.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- Fisher H. W., Yeh J. Contact inhibition in colony formation. Science. 1967 Feb 3;155(3762):581–582. doi: 10.1126/science.155.3762.581. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., Spector W. Migration into an in vitro experimental wound: a comparison of porcine aortic endothelial and smooth muscle cells and the effect of culture irradiation. Am J Pathol. 1981 May;103(2):271–282. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Harland W. A., Smith A. G., Gilbert J. D. Tissue reaction to atheroma lipids. J Pathol. 1973 Dec;111(4):247–253. doi: 10.1002/path.1711110405. [DOI] [PubMed] [Google Scholar]
- Hessler J. R., Morel D. W., Lewis L. J., Chisolm G. M. Lipoprotein oxidation and lipoprotein-induced cytotoxicity. Arteriosclerosis. 1983 May-Jun;3(3):215–222. doi: 10.1161/01.atv.3.3.215. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Mahfouz M., Travis B. D., Yoss N. L., Keenan M. J. The effect of membrane lipid composition on the permeability of membranes to Ca2+. Ann N Y Acad Sci. 1983;414:44–56. doi: 10.1111/j.1749-6632.1983.tb31673.x. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Consequences of blocked sterol synthesis in cultured cells. DNA synthesis and membrane composition. J Biol Chem. 1977 Jan 25;252(2):409–415. [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W., Heiniger H. J. Biological activity of some oxygenated sterols. Science. 1978 Aug 11;201(4355):498–501. doi: 10.1126/science.663671. [DOI] [PubMed] [Google Scholar]
- MacDougall J. D., Biswas S., Cook R. P. The effects of certain C27 steroids on organ cultures of rabbit aorta. Br J Exp Pathol. 1965 Oct;46(5):549–553. [PMC free article] [PubMed] [Google Scholar]
- Mitchinson M. J. Macrophages, oxidised lipids and atherosclerosis. Med Hypotheses. 1983 Oct;12(2):171–178. doi: 10.1016/0306-9877(83)90079-8. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Nakao J., Ooyama T., Ito H., Chang W. C., Murota S. Comparative effect of lipoxygenase products of arachidonic acid on rat aortic smooth muscle cell migration. Atherosclerosis. 1982 Sep;44(3):339–342. doi: 10.1016/0021-9150(82)90008-9. [DOI] [PubMed] [Google Scholar]
- Naseem S. M., Heald F. P. Cytotoxicity of cholesterol oxides and their effects on cholesterol metabolism in cultured human aortic smooth muscle cells. Biochem Int. 1987 Jan;14(1):71–84. [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. K., Taylor C. B., Tham P., Werthessen N. T., Mikkelson B. Effect of auto-oxidation products from cholesterol on aortic smooth muscle cells: an in vitro study. Arch Pathol Lab Med. 1978 Feb;102(2):57–61. [PubMed] [Google Scholar]
- Peng S. K., Tham P., Taylor C. B., Mikkelson B. Cytotoxicity of oxidation derivatives of cholesterol on cultured aortic smooth muscle cells and their effect on cholesterol biosynthesis. Am J Clin Nutr. 1979 May;32(5):1033–1042. doi: 10.1093/ajcn/32.5.1033. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridolfi R. L., Hutchins G. M. The relationship between coronary artery lesions and myocardial infarcts: ulceration of atherosclerotic plaques precipitating coronary thrombosis. Am Heart J. 1977 Apr;93(4):468–486. doi: 10.1016/s0002-8703(77)80410-9. [DOI] [PubMed] [Google Scholar]
- Schroepfer G. J., Jr Sterol biosynthesis. Annu Rev Biochem. 1981;50:585–621. doi: 10.1146/annurev.bi.50.070181.003101. [DOI] [PubMed] [Google Scholar]
- Sholley M. M., Gimbrone M. A., Jr, Cotran R. S. Cellular migration and replication in endothelial regeneration: a study using irradiated endothelial cultures. Lab Invest. 1977 Jan;36(1):18–25. [PubMed] [Google Scholar]
- Smith E. B. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12(0):1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Smith L. L. Cholesterol autoxidation 1981-1986. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):87–125. doi: 10.1016/0009-3084(87)90046-6. [DOI] [PubMed] [Google Scholar]
- Smith L. L., Van Lier J. E. Sterol metabolism. 9.26-hydroxycholesterol levels in the human aorta. Atherosclerosis. 1970 Jul-Aug;12(1):1–14. doi: 10.1016/0021-9150(70)90078-x. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Strom S. C., Michalopoulos G. Collagen as a substrate for cell growth and differentiation. Methods Enzymol. 1982;82(Pt A):544–555. doi: 10.1016/0076-6879(82)82086-7. [DOI] [PubMed] [Google Scholar]
- Taylor F. R., Kandutsch A. A. Oxysterol binding protein. Chem Phys Lipids. 1985 Aug 30;38(1-2):187–194. doi: 10.1016/0009-3084(85)90066-0. [DOI] [PubMed] [Google Scholar]
- Tracy R. E., Strong J. P., Toca V. T., Lopez C. R. Atheronecrosis and its fibroproliferative base and cap in the thoracic aorta. Lab Invest. 1979 Dec;41(6):546–552. [PubMed] [Google Scholar]
- Van Lier J. E., Smith L. L. Sterol metabolism. I. 26-Hydroxycholesterol in the human aorta. Biochemistry. 1967 Oct;6(10):3269–3278. doi: 10.1021/bi00862a037. [DOI] [PubMed] [Google Scholar]
- Vogel A., Ross R., Raines E. Role of serum components in density-dependent inhibition of growth of cells in culture. Platelet-derived growth factor is the major serum determinant of saturation density. J Cell Biol. 1980 May;85(2):377–385. doi: 10.1083/jcb.85.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]