Abstract
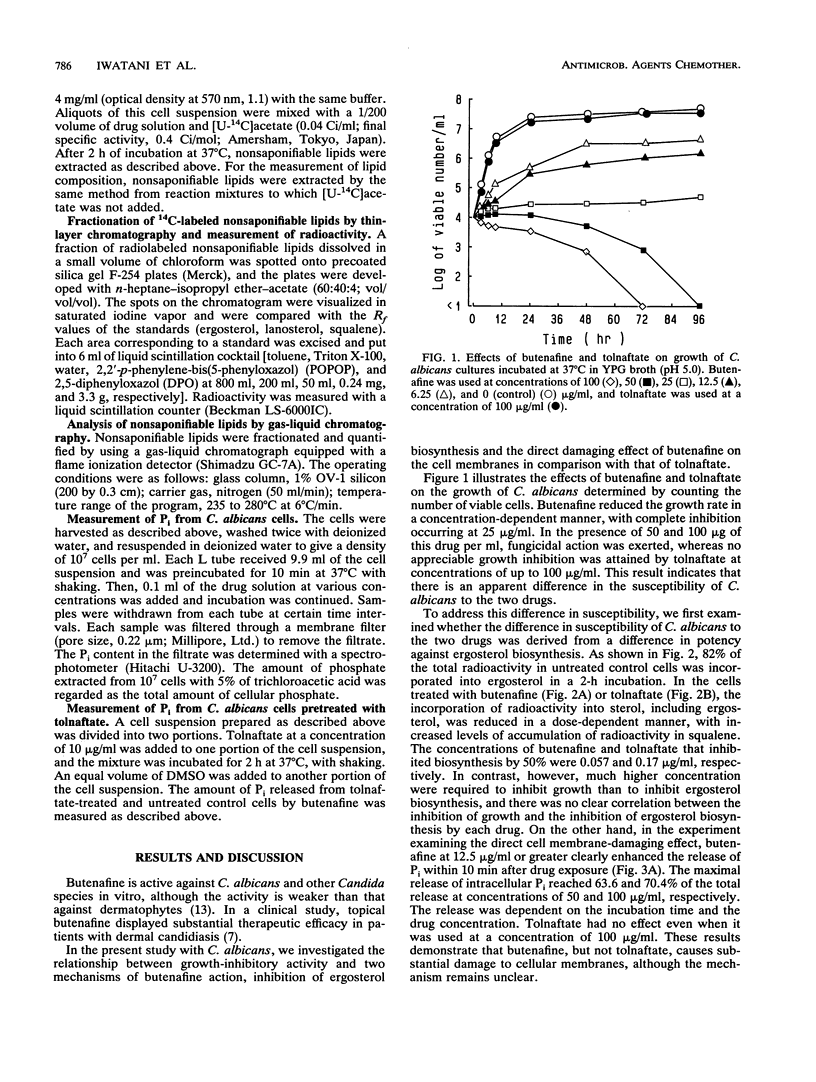
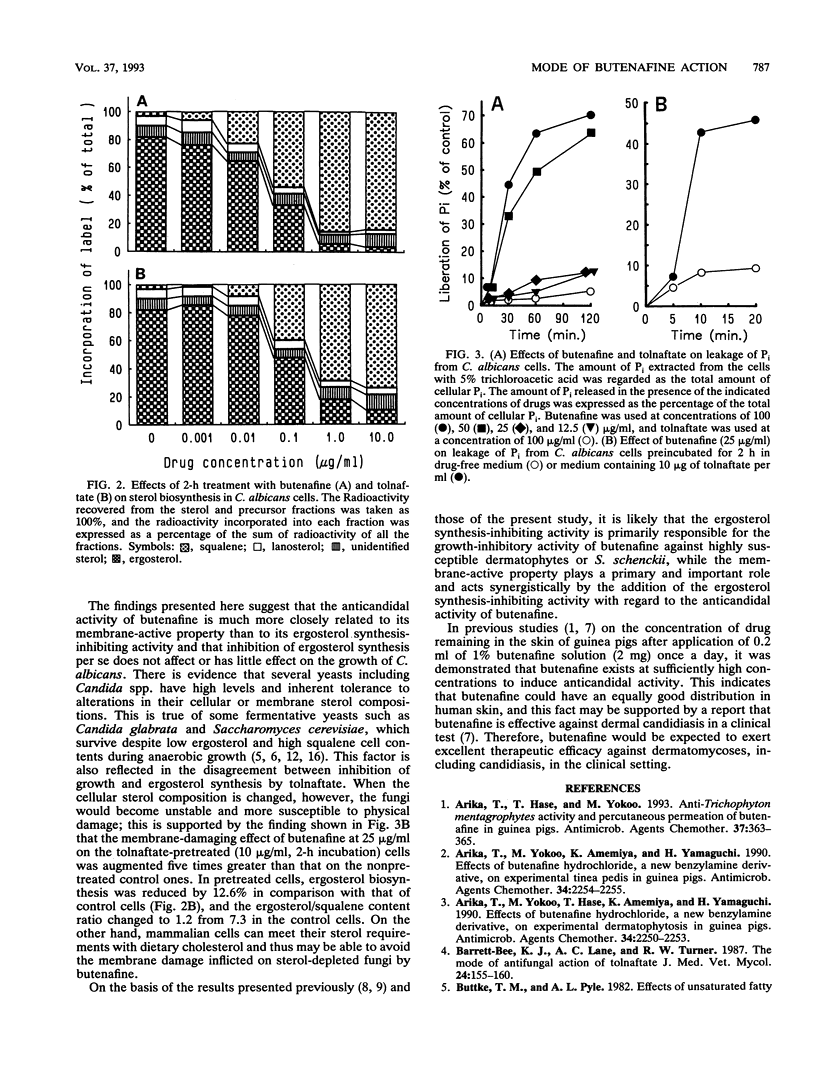
The mechanism of action of a new benzylamine antimycotic, butenafine hydrochloride, was studied in Candida albicans by using the thiocarbamate antimycotic tolnaftate as a reference drug. Butenafine completely inhibited the growth of a test strain of C. albicans at 25 micrograms/ml and was cidal at 50 micrograms/ml. Tolnaftate did not show any growth-inhibitory activity up to 100 micrograms/ml. Both butenafine and tolnaftate inhibited squalene epoxidation in C. albicans, with 50% inhibitory concentrations being 0.57 and 0.17 microgram/ml, respectively. Butenafine, but not tolnaftate, induced the release of appreciable amounts of Pi from C. albicans cells at 12.5 micrograms/ml. This effect of butenafine was augmented when the cells were pretreated with tolnaftate. The results suggest that the direct membrane-damaging effect of butenafine may play a major role in its anticandidal activity and that the drug-induced alteration in the cellular sterol composition renders the cell membrane more susceptible to the membrane-damaging effect of this drug.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arika T., Hase T., Yokoo M. Anti-Trichophyton mentagrophytes activity and percutaneous permeation of butenafine in guinea pigs. Antimicrob Agents Chemother. 1993 Feb;37(2):363–365. doi: 10.1128/aac.37.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arika T., Yokoo M., Hase T., Maeda T., Amemiya K., Yamaguchi H. Effects of butenafine hydrochloride, a new benzylamine derivative, on experimental dermatophytosis in guinea pigs. Antimicrob Agents Chemother. 1990 Nov;34(11):2250–2253. doi: 10.1128/aac.34.11.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arika T., Yokoo M., Maeda T., Amemiya K., Yamaguchi H. Effects of butenafine hydrochloride, a new benzylamine derivative, on experimental tinea pedis in guinea pigs. Antimicrob Agents Chemother. 1990 Nov;34(11):2254–2255. doi: 10.1128/aac.34.11.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Bee K. J., Lane A. C., Turner R. W. The mode of antifungal action of tolnaftate. J Med Vet Mycol. 1986 Apr;24(2):155–160. doi: 10.1080/02681218680000221. [DOI] [PubMed] [Google Scholar]
- Fryberg M., Oehlschlager A. C., Unrau A. M. Biosynthesis of ergosterol in yeast. Evidence for multiple pathways. J Am Chem Soc. 1973 Aug 22;95(17):5747–5757. doi: 10.1021/ja00798a051. [DOI] [PubMed] [Google Scholar]
- KLEIN H. P. Synthesis of lipids in resting cells of Saccharomyces cerevisiae. J Bacteriol. 1955 Jun;69(6):620–627. doi: 10.1128/jb.69.6.620-627.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Takase M., Ishibashi A., Yamamoto T., Sasaki K., Arika T., Yokoo M., Amemiya K. [Synthesis and antifungal activity of butenafine hydrochloride (KP-363), a new benzylamine antifungal agent]. Yakugaku Zasshi. 1991 Feb;111(2):126–137. doi: 10.1248/yakushi1947.111.2_126. [DOI] [PubMed] [Google Scholar]
- Morita T., Nozawa Y. Effects of antifungal agents on ergosterol biosynthesis in Candida albicans and Trichophyton mentagrophytes: differential inhibitory sites of naphthiomate and miconazole. J Invest Dermatol. 1985 Nov;85(5):434–437. doi: 10.1111/1523-1747.ep12277141. [DOI] [PubMed] [Google Scholar]
- Petranyi G., Ryder N. S., Stütz A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science. 1984 Jun 15;224(4654):1239–1241. doi: 10.1126/science.6547247. [DOI] [PubMed] [Google Scholar]
- Ryder N. S., Dupont M. C. Inhibition of squalene epoxidase by allylamine antimycotic compounds. A comparative study of the fungal and mammalian enzymes. Biochem J. 1985 Sep 15;230(3):765–770. doi: 10.1042/bj2300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder N. S., Frank I., Dupont M. C. Ergosterol biosynthesis inhibition by the thiocarbamate antifungal agents tolnaftate and tolciclate. Antimicrob Agents Chemother. 1986 May;29(5):858–860. doi: 10.1128/aac.29.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder N. S., Seidl G., Troke P. F. Effect of the antimycotic drug naftifine on growth of and sterol biosynthesis in Candida albicans. Antimicrob Agents Chemother. 1984 Apr;25(4):483–487. doi: 10.1128/aac.25.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder N. S. Specific inhibition of fungal sterol biosynthesis by SF 86-327, a new allylamine antimycotic agent. Antimicrob Agents Chemother. 1985 Feb;27(2):252–256. doi: 10.1128/aac.27.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]



