Abstract
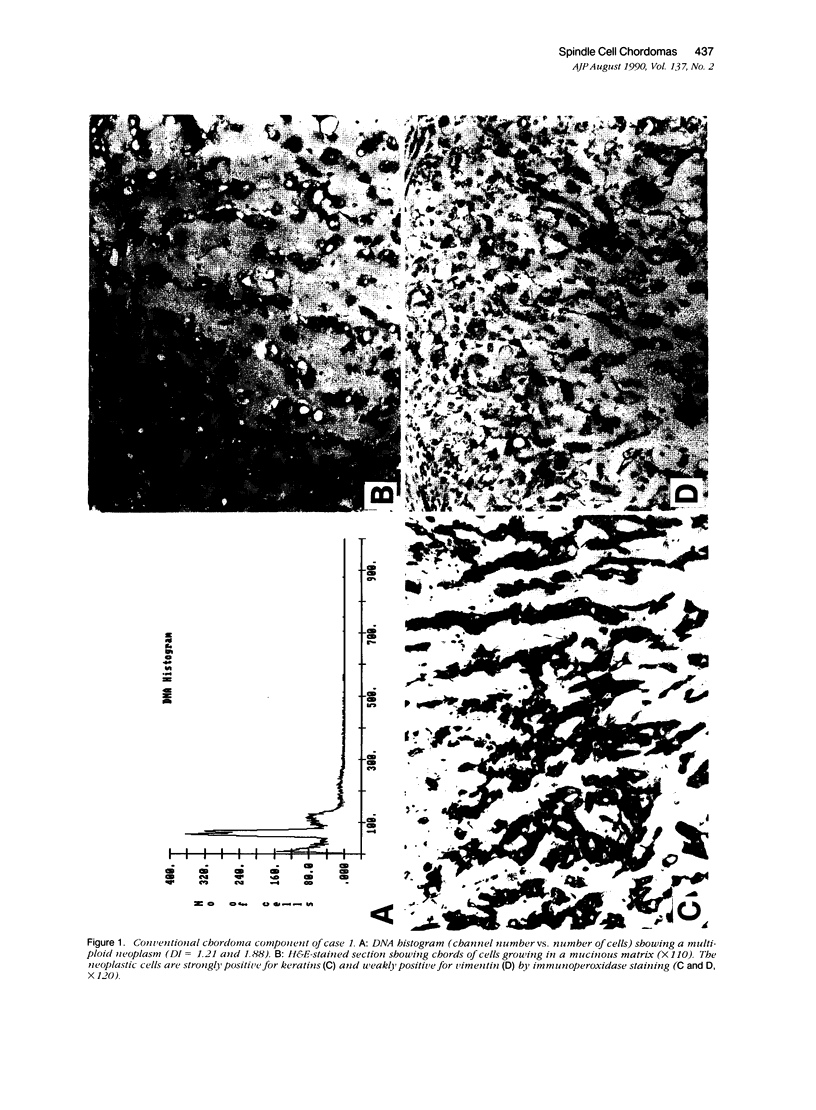
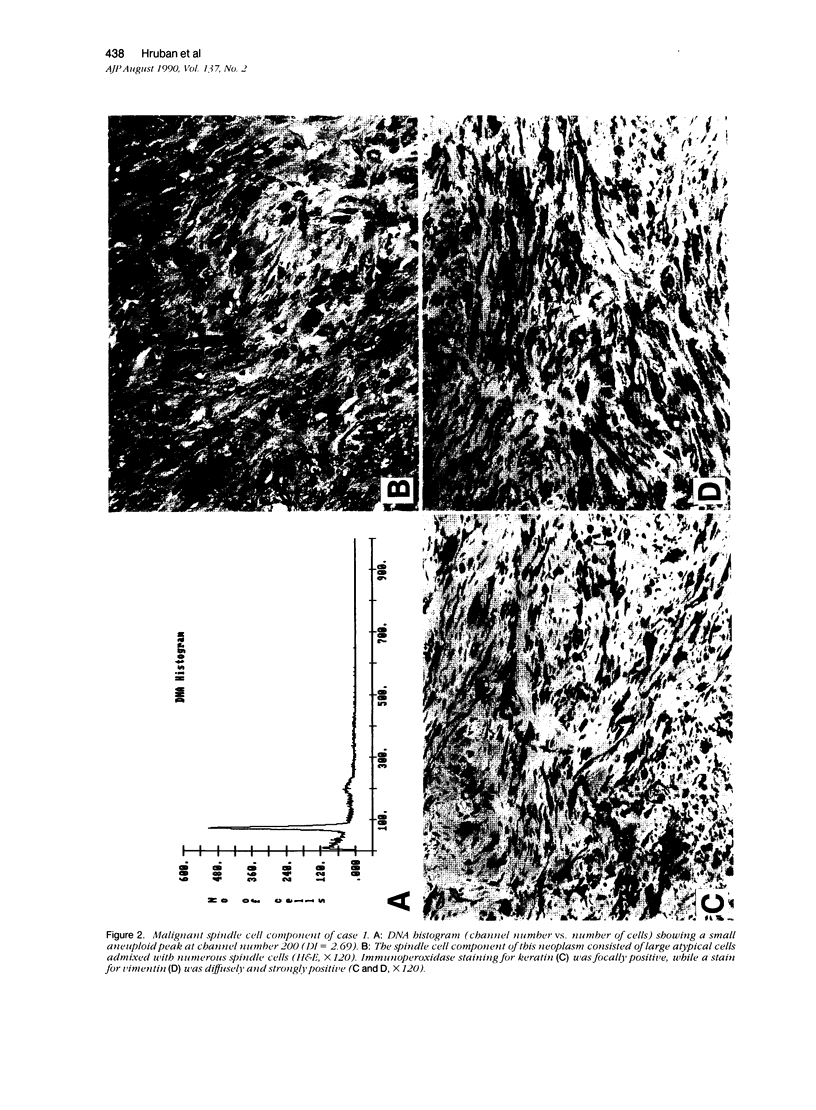
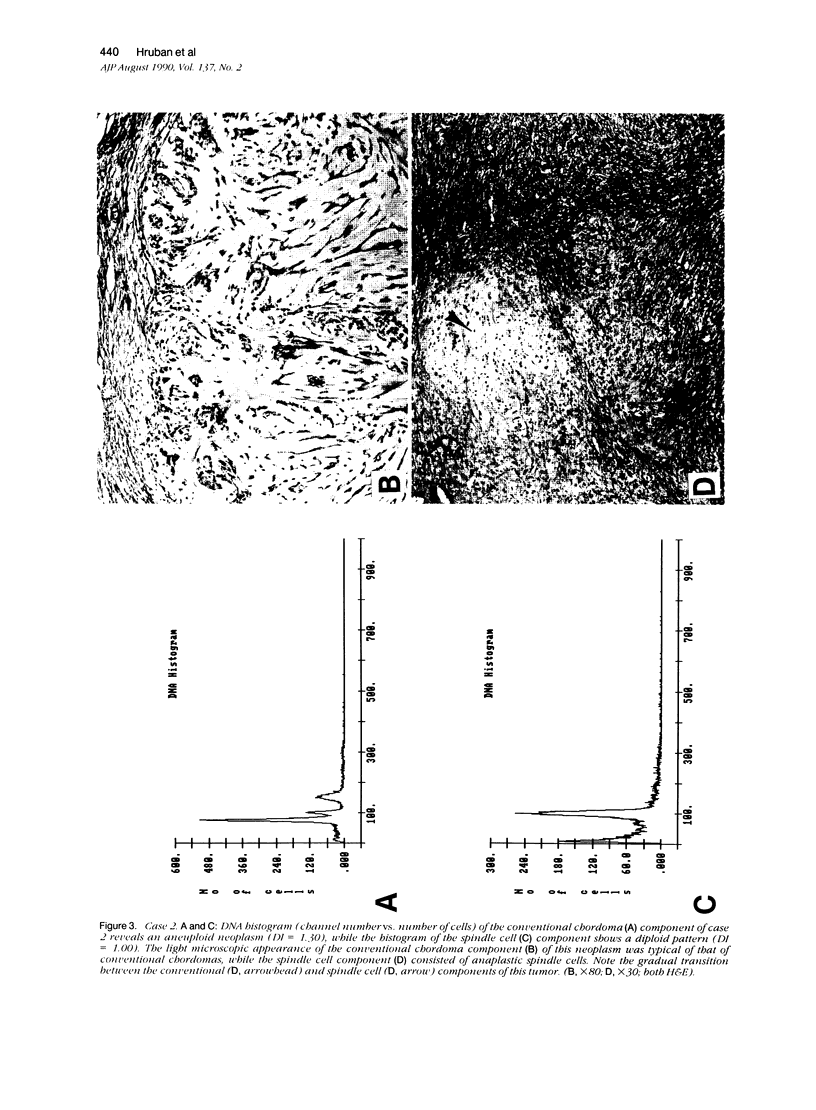
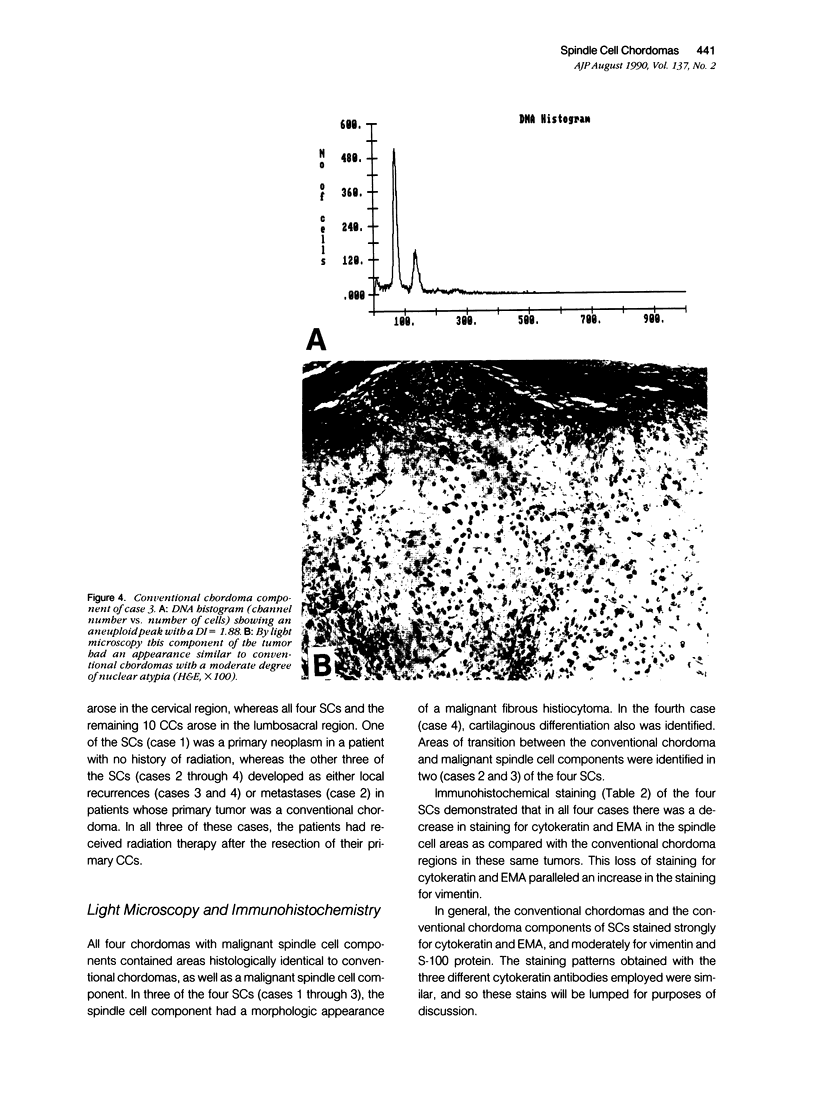
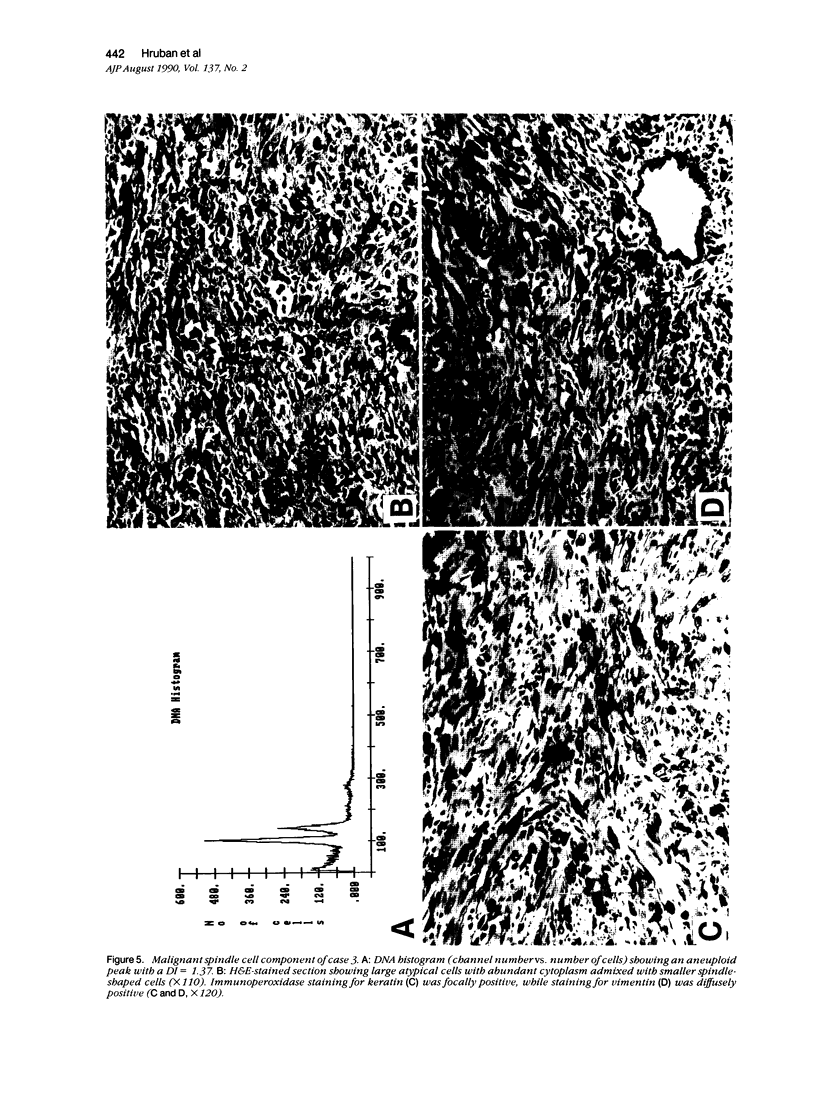
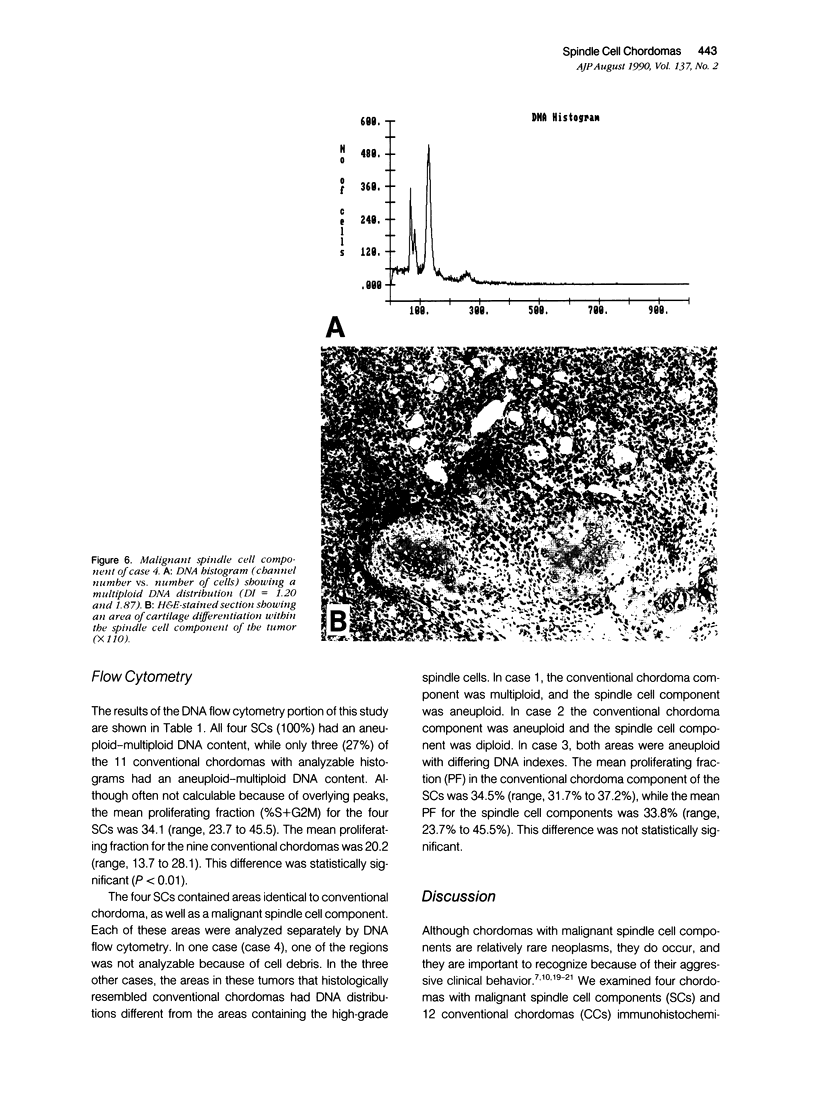
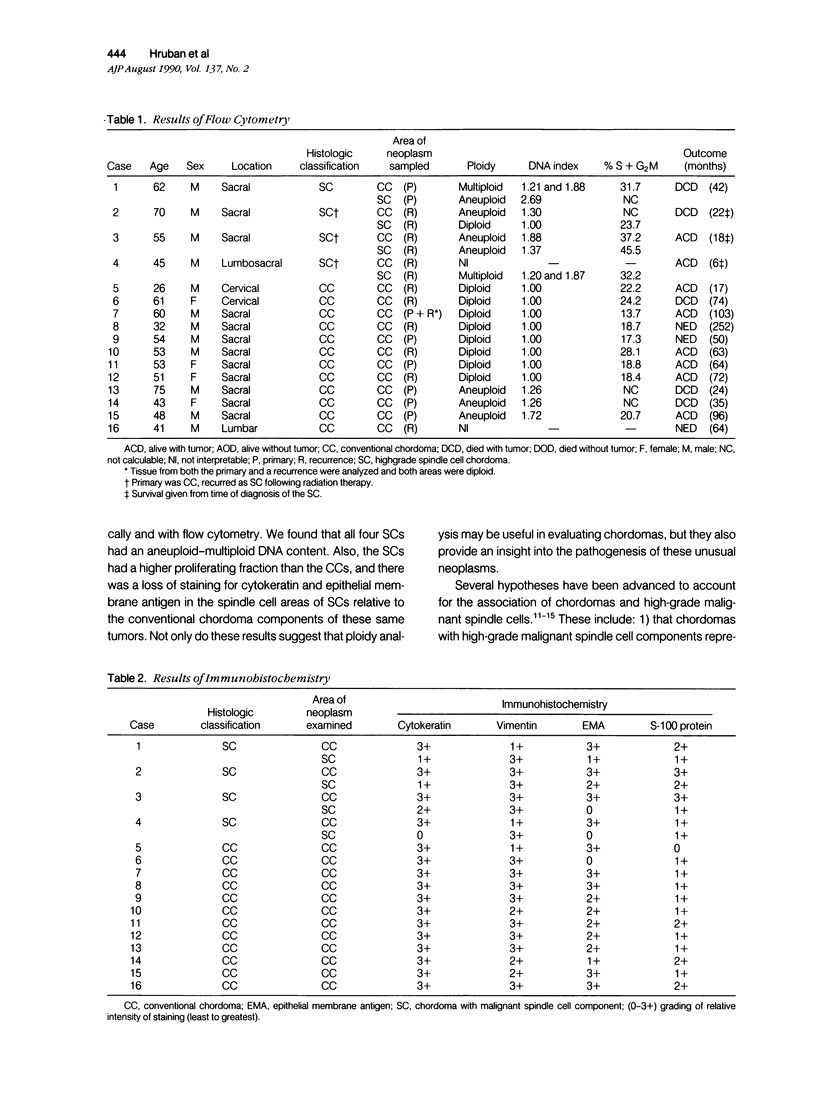
The authors studied four chordomas with malignant spindle cell components (SCs) and 12 conventional chordomas (CCs) by DNA flow cytometry using paraffin-embedded tissue. In addition, immunohistochemical stains for a variety of epithelial and mesenchymal markers were performed. The four SCs contained areas histologically identical to conventional chordomas, as well as a high-grade malignant spindle cell component. All four (100%) SCs had an aneuploid-multiploid DNA content. Of interest, the conventional chordoma areas in these tumors had DNA contents different from those containing the high-grade malignant spindle cells. In contrast, only three (27%) of the 11 conventional chordomas with analyzable histograms had an aneuploid-multiploid DNA content. Immunohistochemical studies performed on the four SCs showed the high-grade malignant spindle cells to stain strongly for vimentin and weakly for cytokeratin, S-100 protein, and epithelial membrane antigen (EMA), whereas the areas of conventional chordoma in these same neoplasms stained moderately for vimentin and S-100 protein, and strongly for cytokeratin and EMA. In two cases, the staining for EMA and cytokeratin highlighted a gradual transition between the areas of conventional chordoma and the spindle cell areas. The immunohistochemical staining pattern of the 12 conventional chordomas was similar to that seen in the conventional chordoma components of the four chordomas with malignant spindle cell components. These results suggest that: 1) aneuploidy is more common in SCs than in CCs, and 2) some SCs are multipotential neoplasms in which the neoplastic cells are capable of differentiation along both epithelial and mesenchymal pathways.
Full text
PDF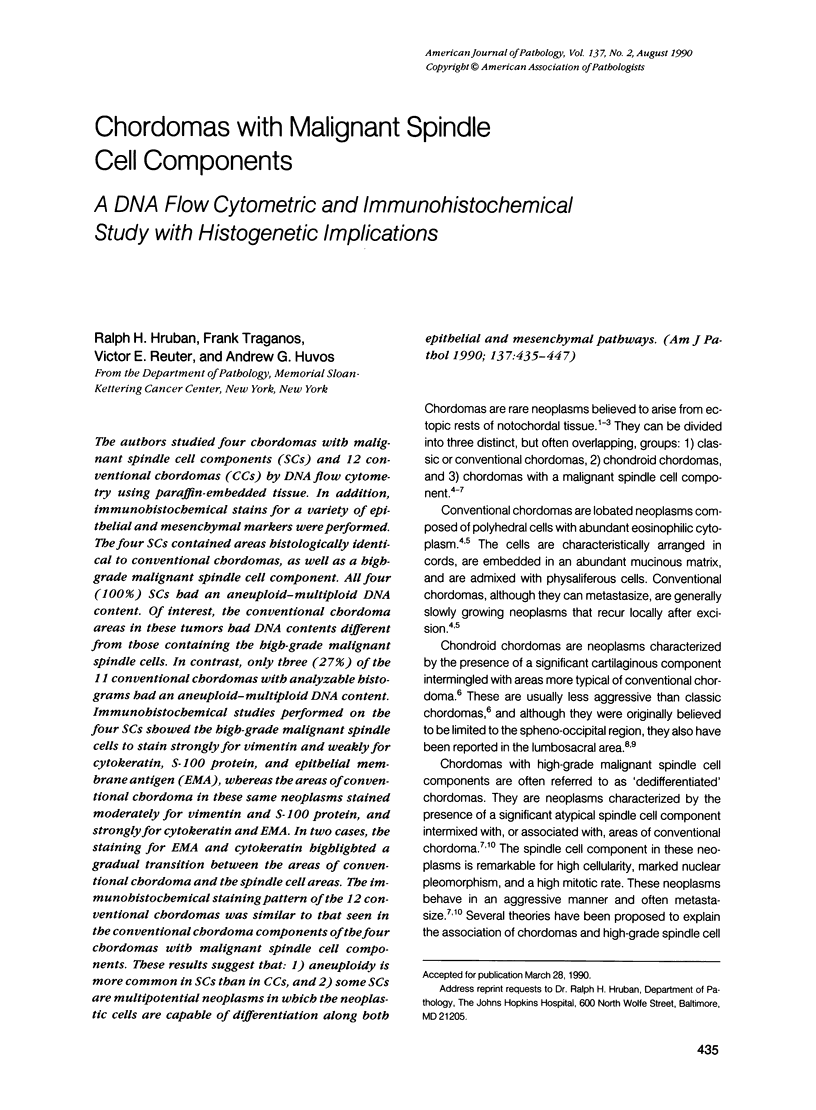





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belza M. G., Urich H. Chordoma and malignant fibrous histiocytoma. Evidence for transformation. Cancer. 1986 Sep 1;58(5):1082–1087. doi: 10.1002/1097-0142(19860901)58:5<1082::aid-cncr2820580517>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Brooks J. J. The significance of double phenotypic patterns and markers in human sarcomas. A new model of mesenchymal differentiation. Am J Pathol. 1986 Oct;125(1):113–123. [PMC free article] [PubMed] [Google Scholar]
- CONGDON C. C. Proliferative lesions resembling chordoma following puncture of the nucleus pulposus in rabbits. J Natl Cancer Inst. 1952 Feb;12(4):893–907. [PubMed] [Google Scholar]
- Chambers P. W., Schwinn C. P. Chordoma. A clinicopathologic study of metastasis. Am J Clin Pathol. 1979 Nov;72(5):765–776. doi: 10.1093/ajcp/72.5.765. [DOI] [PubMed] [Google Scholar]
- Chu T. A. Chondroid chordoma of the sacrococcygeal region. Arch Pathol Lab Med. 1987 Sep;111(9):861–864. [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- DAHLIN D. C., MACCARTY C. S. Chordoma. Cancer. 1952 Nov;5(6):1170–1178. doi: 10.1002/1097-0142(195211)5:6<1170::aid-cncr2820050613>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Batsakis J. G., Owano L. R. Unusual manifestations of chordoma. A report of two cases. J Bone Joint Surg Am. 1968 Dec;50(8):1618–1628. [PubMed] [Google Scholar]
- Halpern J., Kopolovic J., Catane R. Malignant fibrous histiocytoma developing in irradiated sacral chordoma. Cancer. 1984 Jun 15;53(12):2661–2662. doi: 10.1002/1097-0142(19840615)53:12<2661::aid-cncr2820531215>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Harwick R. D., Miller A. S. Craniocervical chordomas. Am J Surg. 1979 Oct;138(4):512–516. doi: 10.1016/0002-9610(79)90410-0. [DOI] [PubMed] [Google Scholar]
- Heaton J. M., Turner D. R. Reflections on notochordal differentiation arising from a study of chordomas. Histopathology. 1985 May;9(5):543–550. doi: 10.1111/j.1365-2559.1985.tb02835.x. [DOI] [PubMed] [Google Scholar]
- Hedley D. W. Flow cytometry using paraffin-embedded tissue: five years on. Cytometry. 1989 May;10(3):229–241. doi: 10.1002/cyto.990100302. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Heffelfinger M. J., Dahlin D. C., MacCarty C. S., Beabout J. W. Chordomas and cartilaginous tumors at the skull base. Cancer. 1973 Aug;32(2):410–420. doi: 10.1002/1097-0142(197308)32:2<410::aid-cncr2820320219>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Higinbotham N. L., Phillips R. F., Farr H. W., Hustu H. O. Chordoma. Thirty-five-year study at Memorial Hospital. Cancer. 1967 Nov;20(11):1841–1850. doi: 10.1002/1097-0142(196711)20:11<1841::aid-cncr2820201107>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Hruban R. H., May M., Marcove R. C., Huvos A. G. Lumbo-sacral chordoma with high-grade malignant cartilaginous and spindle cell components. Am J Surg Pathol. 1990 Apr;14(4):384–389. doi: 10.1097/00000478-199004000-00012. [DOI] [PubMed] [Google Scholar]
- Joensuu H., Kallioniemi O. P. Different opinions on classification of DNA histograms produced from paraffin-embedded tissue. Cytometry. 1989 Nov;10(6):711–717. doi: 10.1002/cyto.990100607. [DOI] [PubMed] [Google Scholar]
- Kishikawa H., Tanaka K. Chordoma--report of an autopsy case with fibrosarcoma. Acta Pathol Jpn. 1974 Mar;24(2):299–308. doi: 10.1111/j.1440-1827.1974.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Knechtges T. C. Sacrococcygeal chordoma with sarcomatous features (spindle cell metaplasia). Am J Clin Pathol. 1970 May;53(5):612–616. doi: 10.1093/ajcp/53.5.612. [DOI] [PubMed] [Google Scholar]
- Makek M., Leu H. J. Malignant fibrous histiocytoma arising in a recurrent chordoma. Case report and electron microscopic findings. Virchows Arch A Pathol Anat Histol. 1982;397(3):241–250. doi: 10.1007/BF00496567. [DOI] [PubMed] [Google Scholar]
- Meis J. M., Raymond A. K., Evans H. L., Charles R. E., Giraldo A. A. "Dedifferentiated" chordoma. A clinicopathologic and immunohistochemical study of three cases. Am J Surg Pathol. 1987 Jul;11(7):516–525. [PubMed] [Google Scholar]
- Miettinen M., Karaharju E., Järvinen H. Chordoma with a massive spindle-cell sarcomatous transformation. A light- and electron-microscopic and immunohistological study. Am J Surg Pathol. 1987 Jul;11(7):563–570. doi: 10.1097/00000478-198707000-00009. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Malignant fibrous histiocytoma within a recurrent chordoma. A light microscopic, electron microscopic, and immunohistochemical study. Am J Clin Pathol. 1984 Dec;82(6):738–743. doi: 10.1093/ajcp/82.6.738. [DOI] [PubMed] [Google Scholar]
- POPPEN J. L., KING A. B. Chordoma: experience with thirteen cases. J Neurosurg. 1952 Mar;9(2):139–163. doi: 10.3171/jns.1952.9.2.0139. [DOI] [PubMed] [Google Scholar]
- Rone R., Ramzy I., Duncan D. Anaplastic sacrococcygeal chordoma. Fine needle aspiration cytologic findings and embryologic considerations. Acta Cytol. 1986 Mar-Apr;30(2):183–188. [PubMed] [Google Scholar]
- Salisbury J. R., Isaacson P. G. Demonstration of cytokeratins and an epithelial membrane antigen in chordomas and human fetal notochord. Am J Surg Pathol. 1985 Nov;9(11):791–797. doi: 10.1097/00000478-198511000-00002. [DOI] [PubMed] [Google Scholar]
- Volpe R., Mazabraud A. A clinicopathologic review of 25 cases of chordoma (a pleomorphic and metastasizing neoplasm). Am J Surg Pathol. 1983 Mar;7(2):161–170. doi: 10.1097/00000478-198303000-00006. [DOI] [PubMed] [Google Scholar]








