Abstract
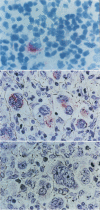
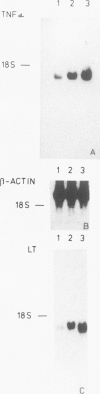
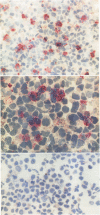
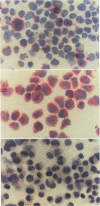
It is likely that the characteristic histologic features of Hodgkin's disease reflect cytokine production by the tumor cell population. Tumor necrosis factor α (TNF-α) and lymphotoxin (tumor necrosis factor beta [TNF-β]) are important inflammatory mediators with wide-ranging effects within the lymphoreticular system. The aim of the present study was to investigate TNF-α and lymphotoxin production in the Hodgkin's disease-derived cell lines L428 and L540. At the product level, both cytokines could be demonstrated by immunostaining with specific monoclonal antibodies. TNF-α could be demonstrated by means of an enzyme-linked immunosorbent assay in culture supernatants from both cell lines as well as in cell lysates of L428 and L540 cells. Cytotoxic activity could be achieved only in L428 supernatants. This cytotoxic activity could not be blocked by the addition of a polyclonal antibody against TNF-α, but was partially inhibited with the monoclonal antibody against lymphotoxin. Synthesis of TNF-α and lymphotoxin in both L428 and L540 was confirmed by demonstrating the intracellular-specific messenger RNA (mRNA) using specific cDNA clones in Northern blot analysis. In situ hybridization studies with the TNF-α cDNA probe gave positive hybridization signals in L428 and in L540. These results demonstrate the transcription, translation, and export of TNF-α and lymphotoxin in cultured Hodgkin's disease-derived cell lines. In addition, results of preliminary experiments are presented in which we demonstrate Reed-Sternberg cells positive for TNF-α protein and mRNA in different Hodgkin's disease tissue biopsies, indicating that, at least for TNF-α, our cell line data are relevant to the neoplastic population present in Hodgkin's disease tissue.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendtzen K. Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Lett. 1988 Nov;19(3):183–191. doi: 10.1016/0165-2478(88)90141-1. [DOI] [PubMed] [Google Scholar]
- Bringman T. S., Aggarwal B. B. Monoclonal antibodies to human tumor necrosis factors alpha and beta: application for affinity purification, immunoassays, and as structural probes. Hybridoma. 1987 Oct;6(5):489–507. doi: 10.1089/hyb.1987.6.489. [DOI] [PubMed] [Google Scholar]
- Budowsky E. I., Sverdlov E. D., Monastyrskaya G. S. New method of selective and rapid modification of the cytidine residues. FEBS Lett. 1972 Sep 1;25(1):201–204. doi: 10.1016/0014-5793(72)80485-x. [DOI] [PubMed] [Google Scholar]
- Burrichter H., Heit W., Schaadt M., Kirchner H., Diehl V. Production of colony-stimulating factors by Hodgkin cell lines. Int J Cancer. 1983 Mar 15;31(3):269–274. doi: 10.1002/ijc.2910310303. [DOI] [PubMed] [Google Scholar]
- Chang R. J., Lee S. H. Effects of interferon-gamma and tumor necrosis factor-alpha on the expression of an Ia antigen on a murine macrophage cell line. J Immunol. 1986 Nov 1;137(9):2853–2856. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Diehl V., Kirchner H. H., Schaadt M., Fonatsch C., Stein H., Gerdes J., Boie C. Hodgkin's disease: establishment and characterization of four in vitro cell lies. J Cancer Res Clin Oncol. 1981;101(1):111–124. doi: 10.1007/BF00405072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H. G., Jones D. B., Diehl V., Minowada J. Is the Hodgkin cell a T- or B-lymphocyte? Recent evidence from geno- and immunophenotypic analysis and in-vitro cell lines. Hematol Oncol. 1989 Mar-Apr;7(2):95–113. doi: 10.1002/hon.2900070202. [DOI] [PubMed] [Google Scholar]
- Ellis T. M., McMannis J. D., Chua A. O., Gubler U., Fisher R. I. Interleukin-1-independent activation of human T lymphocytes stimulated by anti-CD3 and a Hodgkin's disease cell line with accessory cell activity. Cell Immunol. 1988 Oct 15;116(2):352–366. doi: 10.1016/0008-8749(88)90237-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F., Sauder D. N., Scala G., Diehl V. Neoplastic cells obtained from Hodgkin's disease are potent stimulators of human primary mixed lymphocyte cultures. J Immunol. 1983 Jun;130(6):2666–2670. [PubMed] [Google Scholar]
- Ford R. J., Mehta S., Davis F., Maizel A. L. Growth factors in Hodgkin's disease. Cancer Treat Rep. 1982 Apr;66(4):633–638. [PubMed] [Google Scholar]
- Freudenberg N., Joh K., Westphal O., Mittermayer C., Freudenberg M. A., Galanos C. Haemorrhagic tumour necrosis following endotoxin administration. I. Communication: morphological investigation on endotoxin-induced necrosis of the methylcholanthrene (Meth A) tumour in the mouse. Virchows Arch A Pathol Anat Histopathol. 1984;403(4):377–389. doi: 10.1007/BF00737287. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- Herbst H., Tippelmann G., Anagnostopoulos I., Gerdes J., Schwarting R., Boehm T., Pileri S., Jones D. B., Stein H. Immunoglobulin and T-cell receptor gene rearrangements in Hodgkin's disease and Ki-1-positive anaplastic large cell lymphoma: dissociation between phenotype and genotype. Leuk Res. 1989;13(2):103–116. doi: 10.1016/0145-2126(89)90134-3. [DOI] [PubMed] [Google Scholar]
- Hurme M. Both interleukin 1 and tumor necrosis factor enhance thymocyte proliferation. Eur J Immunol. 1988 Aug;18(8):1303–1306. doi: 10.1002/eji.1830180824. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Furley A. J., Gerdes J., Greaves M. F., Stein H., Wright D. H. Phenotypic and genotypic analysis of two cell lines derived from Hodgkin's disease tissue biopsies. Recent Results Cancer Res. 1989;117:62–66. doi: 10.1007/978-3-642-83781-4_6. [DOI] [PubMed] [Google Scholar]
- Jones D. B. The histogenesis of the Reed-Sternberg cell and its mononuclear counterparts. J Pathol. 1987 Mar;151(3):191–195. doi: 10.1002/path.1711510306. [DOI] [PubMed] [Google Scholar]
- Kadin M. E. A reappraisal of the Reed-Sternberg cell: a commentary. Blood Cells. 1980;6(3):525–532. [PubMed] [Google Scholar]
- Kehrl J. H., Miller A., Fauci A. S. Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J Exp Med. 1987 Sep 1;166(3):786–791. doi: 10.1084/jem.166.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortmann C., Burrichter H., Monner D., Jahn G., Diehl V., Peter H. H. Interleukin-1-like activity constitutively generated by Hodgkin derived cell lines. I. Measurement in a human lymphocyte co-stimulator assay. Immunobiology. 1984 May;166(3):318–333. doi: 10.1016/s0171-2985(84)80049-2. [DOI] [PubMed] [Google Scholar]
- Last-Barney K., Homon C. A., Faanes R. B., Merluzzi V. J. Synergistic and overlapping activities of tumor necrosis factor-alpha and IL-1. J Immunol. 1988 Jul 15;141(2):527–530. [PubMed] [Google Scholar]
- Meager A., Leung H., Woolley J. Assays for tumour necrosis factor and related cytokines. J Immunol Methods. 1989 Jan 6;116(1):1–17. doi: 10.1016/0022-1759(89)90306-2. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Ansari A. A., Diehl V. L-428 nodular sclerosing Hodgkin's cell secretes a unique transforming growth factor-beta active at physiologic pH. J Clin Invest. 1988 Dec;82(6):1915–1921. doi: 10.1172/JCI113810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Ansari A. A. Production of transforming growth factor-beta activity by Ki-1 positive lymphoma cells and analysis of its role in the regulation of Ki-1 positive lymphoma growth. Am J Pathol. 1988 Jun;131(3):569–577. [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., O'Rourke L. Potentiation of fibroblast growth by nodular sclerosing Hodgkin's disease cell cultures. Blood. 1982 Jul;60(1):228–237. [PubMed] [Google Scholar]
- Ostensen M. E., Thiele D. L., Lipsky P. E. Tumor necrosis factor-alpha enhances cytolytic activity of human natural killer cells. J Immunol. 1987 Jun 15;138(12):4185–4191. [PubMed] [Google Scholar]
- Paul N. L., Ruddle N. H. Lymphotoxin. Annu Rev Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Perussia B., Kobayashi M., Rossi M. E., Anegon I., Trinchieri G. Immune interferon enhances functional properties of human granulocytes: role of Fc receptors and effect of lymphotoxin, tumor necrosis factor, and granulocyte-macrophage colony-stimulating factor. J Immunol. 1987 Feb 1;138(3):765–774. [PubMed] [Google Scholar]
- Pober J. S., Lapierre L. A., Stolpen A. H., Brock T. A., Springer T. A., Fiers W., Bevilacqua M. P., Mendrick D. L., Gimbrone M. A., Jr Activation of cultured human endothelial cells by recombinant lymphotoxin: comparison with tumor necrosis factor and interleukin 1 species. J Immunol. 1987 May 15;138(10):3319–3324. [PubMed] [Google Scholar]
- Powell M. B., Conta B. S., Horowitz M., Ruddle N. H. The differential inhibitory effect of lymphotoxin and immune interferon on normal and malignant lymphoid cells. Lymphokine Res. 1985 Winter;4(1):13–26. [PubMed] [Google Scholar]
- Pringle J. H., Homer C. E., Warford A., Kendall C. H., Lauder I. In situ hybridization: alkaline phosphatase visualization of biotinylated probes in cryostat and paraffin sections. Histochem J. 1987 Sep;19(9):488–496. doi: 10.1007/BF01675419. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Ruggiero V., Baglioni C. Synergistic anti-proliferative activity of interleukin 1 and tumor necrosis factor. J Immunol. 1987 Feb 1;138(3):661–663. [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Stefanini M., De Martino C., Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967 Oct 14;216(5111):173–174. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Gatter K., Asbahr H., Mason D. Y. Use of freeze-dried paraffin-embedded sections for immunohistologic staining with monoclonal antibodies. Lab Invest. 1985 Jun;52(6):676–683. [PubMed] [Google Scholar]
- Stein H., Gerdes J. Phänotypische und genotypische Marker bei malignen Lymphomen: Ein Beitrag zum zellulären Ursprung des Morbus Hodgkin und der malignen Histiozytose sowie Implikationen für die Klassifikation der T-Zell- und B-Zell-Lymphome. Verh Dtsch Ges Pathol. 1986;70:127–151. [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Bonavida B. Studies on the mechanism of natural killer cell-mediated cytotoxicity. VII. functional comparison of human natural killer cytotoxic factors with recombinant lymphotoxin and tumor necrosis factor. J Immunol. 1987 Mar 15;138(6):1791–1798. [PubMed] [Google Scholar]