Abstract
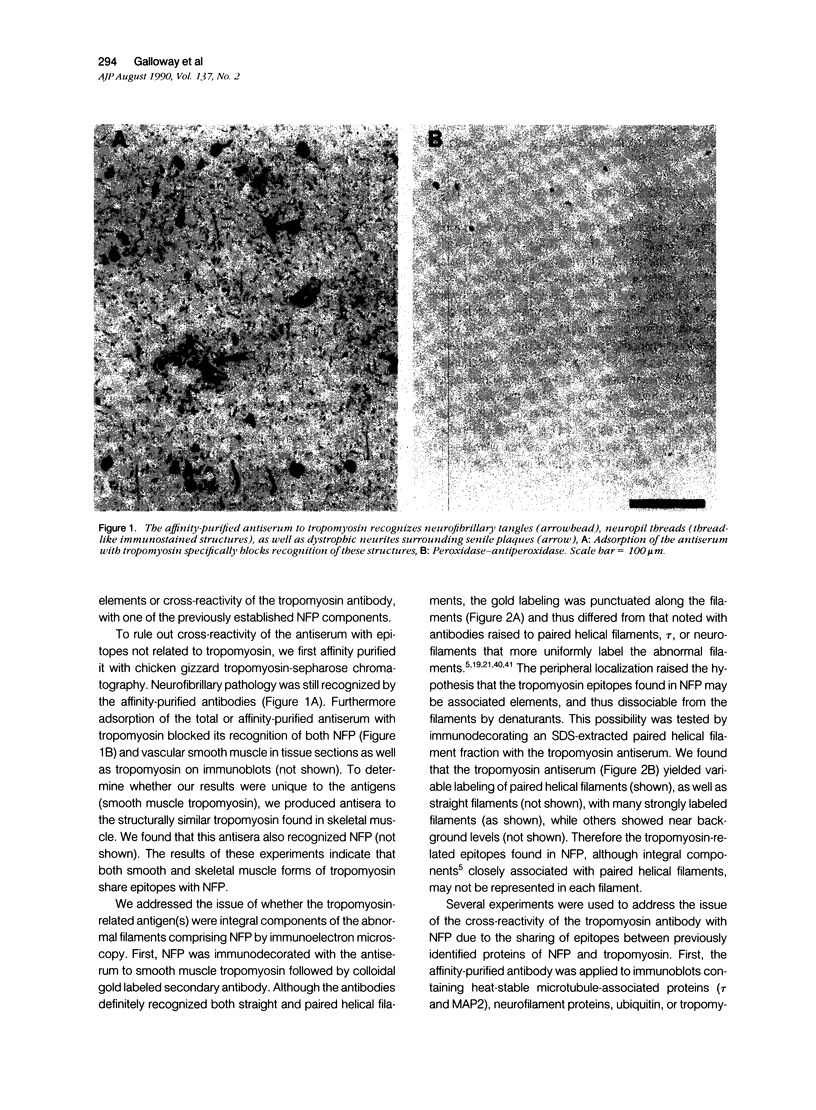
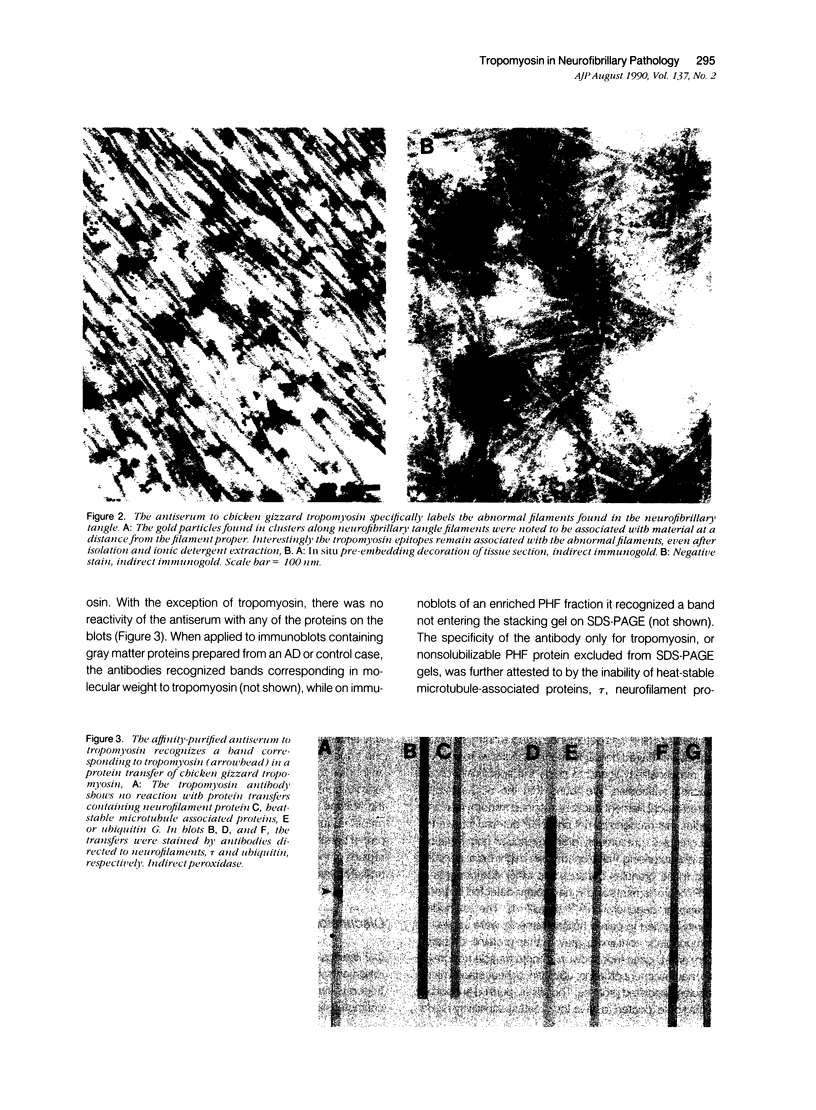
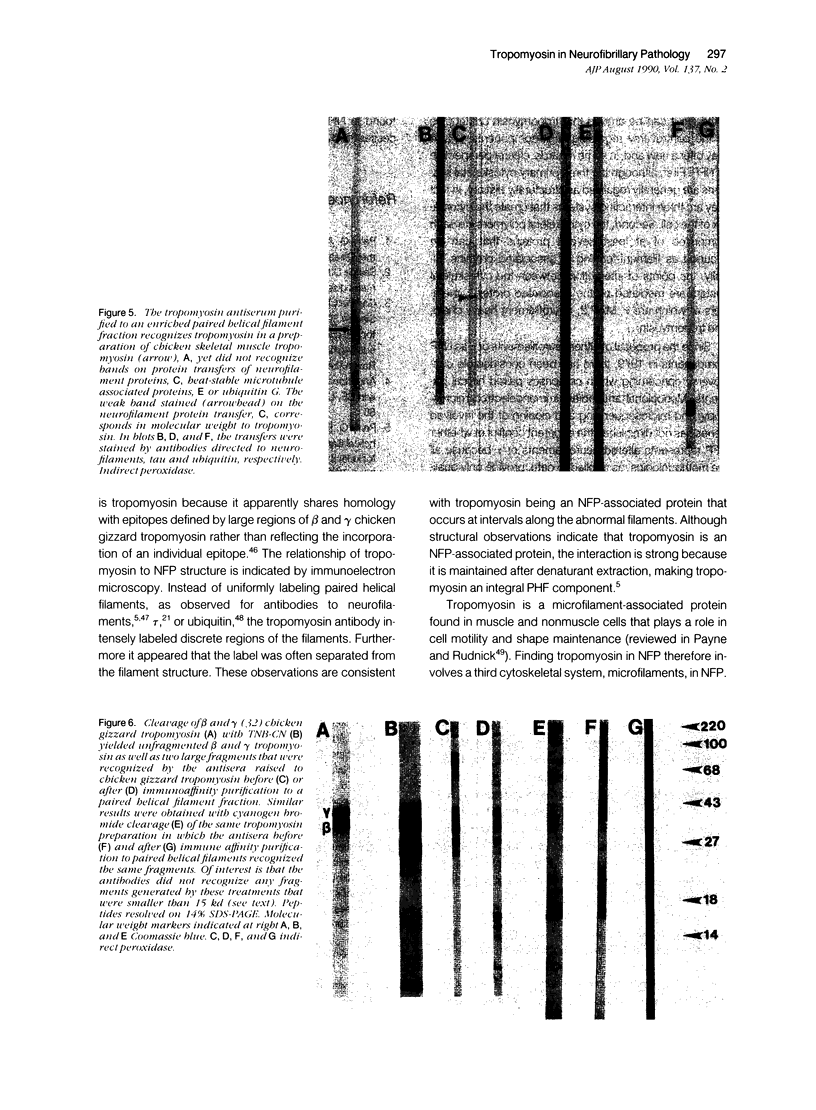
The focus of research on the neurofibrillary pathology (NFP) of Alzheimer disease has been not only to determine the component forming the paired helical filaments but also to determine whether they result from abnormal processes affecting a single protein. Therefore, although these studies have lead to controversy concerning the respective contribution of components of microtubules and neurofilaments, there has been essentially no consideration of whether other cytoskeletal systems might also be involved and of what are the common features for the incorporated components. Particularly relevant to this issue is our finding that several antisera raised to either skeletal or smooth muscle tropomyosin, a microfilament component, intensely recognize NFP. These antibodies continue to recognize NFP after affinity purification to tropomyosin or paired helical filament fractions. We show that the antibodies do not recognize NFP due to cross-reactivity with the previously identified NFP components related to neurofilaments and microtubules, tau, and MAP2, or neurofilament proteins because the antisera did not recognize these proteins on immunoblots or were not adsorbable by the proteins. Ultrastructural analysis of the immunoreaction showed that tropomyosin-related epitopes were clustered rather than uniformly distributed along paired helical and straight filaments. Although the distribution suggests that tropomyosin is an NFP-associated protein, its retention by paired helical and straight filaments after detergent extraction indicates that it is an integral component strongly and specifically associated with the filaments characteristic of NFP. These findings indicate that NFP involves the three primary neuronal cytoskeletal filament systems, microtubules, neurofilaments, as well as microfilaments, and therefore that NFP probably results from the reorganization of these normal filaments that interact to comprise the cytomatrix and may continue this interaction under the pathologic condition of Alzheimer's disease to generate novel, abnormal polymers.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Autilio-Gambetti L., Sipple J., Sudilovsky O., Gambetti P. Intermediate filaments of Schwann cells. J Neurochem. 1982 Mar;38(3):774–780. doi: 10.1111/j.1471-4159.1982.tb08698.x. [DOI] [PubMed] [Google Scholar]
- Blose S. H., Matsumura F., Lin J. J. Structure of vimentin 10-nm filaments probed with a monoclonal antibody that recognizes a common antigenic determinant on vimentin and tropomyosin. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):455–463. doi: 10.1101/sqb.1982.046.01.042. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Ksiezak-Reding H., Davies P., Yen S. H. A monoclonal antibody that recognizes a phosphorylated epitope in Alzheimer neurofibrillary tangles, neurofilaments and tau proteins immunostains granulovacuolar degeneration. Acta Neuropathol. 1987;73(3):254–258. doi: 10.1007/BF00686619. [DOI] [PubMed] [Google Scholar]
- Galloway P. G., Perry G., Gambetti P. Hirano body filaments contain actin and actin-associated proteins. J Neuropathol Exp Neurol. 1987 Mar;46(2):185–199. doi: 10.1097/00005072-198703000-00006. [DOI] [PubMed] [Google Scholar]
- Galloway P. G., Perry G., Kosik K. S., Gambetti P. Hirano bodies contain tau protein. Brain Res. 1987 Feb 17;403(2):337–340. doi: 10.1016/0006-8993(87)90071-0. [DOI] [PubMed] [Google Scholar]
- Goldman J. E. The association of actin with Hirano bodies. J Neuropathol Exp Neurol. 1983 Mar;42(2):146–152. doi: 10.1097/00005072-198303000-00004. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y. C., Zaidi M. S., Wisniewski H. M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986 May 5;261(13):6084–6089. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Johnson A. B., Wisniewski H. M., Terry R. D., Iqbal K. Evidence that Alzheimer neurofibrillary tangles originate from neurotubules. Lancet. 1979 Mar 17;1(8116):578–580. doi: 10.1016/s0140-6736(79)91006-7. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J., Bershadsky A. D., Pletjushkina O. Y., Tint I. S., Vasiliev J. M. Intermediate filament collapse is an ATP-dependent and actin-dependent process. J Cell Sci. 1989 Apr;92(Pt 4):621–631. doi: 10.1242/jcs.92.4.621. [DOI] [PubMed] [Google Scholar]
- Iqbal K., Zaidi T., Thompson C. H., Merz P. A., Wisniewski H. M. Alzheimer paired helical filaments: bulk isolation, solubility, and protein composition. Acta Neuropathol. 1984;62(3):167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- Kahn J., Anderton B. H., Probst A., Ulrich J., Esiri M. M. Immunohistological study of granulovacuolar degeneration using monoclonal antibodies to neurofilaments. J Neurol Neurosurg Psychiatry. 1985 Sep;48(9):924–926. doi: 10.1136/jnnp.48.9.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Duffy L. K., Dowling M. M., Abraham C., McCluskey A., Selkoe D. J. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Two monoclonal antibodies recognize Alzheimer's neurofibrillary tangles, neurofilament, and microtubule-associated proteins. J Neurochem. 1987 Feb;48(2):455–462. doi: 10.1111/j.1471-4159.1987.tb04114.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manetto V., Perry G., Tabaton M., Mulvihill P., Fried V. A., Smith H. T., Gambetti P., Autilio-Gambetti L. Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Yamashiro-Matsumura S. Purification and characterization of multiple isoforms of tropomyosin from rat cultured cells. J Biol Chem. 1985 Nov 5;260(25):13851–13859. [PubMed] [Google Scholar]
- Miller C. C., Brion J. P., Calvert R., Chin T. K., Eagles P. A., Downes M. J., Flament-Durand J., Haugh M., Kahn J., Probst A. Alzheimer's paired helical filaments share epitopes with neurofilament side arms. EMBO J. 1986 Feb;5(2):269–276. doi: 10.1002/j.1460-2075.1986.tb04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nukina N., Kosik K. S., Selkoe D. J. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci U S A. 1987 May;84(10):3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorato M., Mulvihill P., Connolly J., Galloway P., Whitehouse P., Perry G. Alteration of neuritic cytoarchitecture in Alzheimer disease. Prog Clin Biol Res. 1989;317:781–789. [PubMed] [Google Scholar]
- Payne M. R., Rudnick S. E. Tropomyosin. Structural and functional diversity. Cell Muscle Motil. 1985;6:141–184. [PubMed] [Google Scholar]
- Perry G., Friedman R., Kang D. H., Manetto V., Autilio-Gambetti L., Gambetti P. Antibodies to the neuronal cytoskeleton are elicited by Alzheimer paired helical filament fractions. Brain Res. 1987 Sep 15;420(2):233–242. doi: 10.1016/0006-8993(87)91243-1. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Mulvihill P., Manetto V., Autilio-Gambetti L., Gambetti P. Immunocytochemical properties of Alzheimer straight filaments. J Neurosci. 1987 Nov;7(11):3736–3738. doi: 10.1523/JNEUROSCI.07-11-03736.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Selkoe D. J., Block B. R., Stewart D., Autilio-Gambetti L., Gambetti P. Electron microscopic localization of Alzheimer neurofibrillary tangle components recognized by an antiserum to paired helical filaments. J Neuropathol Exp Neurol. 1986 Mar;45(2):161–168. doi: 10.1097/00005072-198603000-00006. [DOI] [PubMed] [Google Scholar]
- Peterson C., Kress Y., Vallee R., Goldman J. E. High molecular weight microtubule-associated proteins bind to actin lattices (Hirano bodies). Acta Neuropathol. 1988;77(2):168–174. doi: 10.1007/BF00687427. [DOI] [PubMed] [Google Scholar]
- Sanders C., Burtnick L. D., Smillie L. B. Native chicken gizzard tropomyosin is predominantly a beta gamma-heterodimer. J Biol Chem. 1986 Sep 25;261(27):12774–12778. [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Selkoe D. J. Biochemistry of altered brain proteins in Alzheimer's disease. Annu Rev Neurosci. 1989;12:463–490. doi: 10.1146/annurev.ne.12.030189.002335. [DOI] [PubMed] [Google Scholar]
- Shecket G., Lasek R. J. Preparation of neurofilament protein from guinea pig peripheral nerve and spinal cord. J Neurochem. 1980 Dec;35(6):1335–1344. doi: 10.1111/j.1471-4159.1980.tb09007.x. [DOI] [PubMed] [Google Scholar]
- Slepecky N., Chamberlain S. C. Tropomyosin co-localizes with actin microfilaments and microtubules within supporting cells of the inner ear. Cell Tissue Res. 1987 Apr;248(1):63–66. doi: 10.1007/BF01239963. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H. Local structural changes in tropomyosin detected by a trypsin-probe method. Biochemistry. 1984 Sep 25;23(20):4791–4798. doi: 10.1021/bi00315a040. [DOI] [PubMed] [Google Scholar]
- Ueno H., Takahashi S., Ooi T. Tropomyosin fragments cleaved at the cysteinyl residue. J Biochem. 1977 Jul;82(1):131–138. doi: 10.1093/oxfordjournals.jbchem.a131661. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Dickson D. W., Crowe A., Butler M., Shelanski M. L. Alzheimer's neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubule associated proteins tau and MAP2. Am J Pathol. 1987 Jan;126(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Gaskin F., Terry R. D. Immunocytochemical studies of neurofibrillary tangles. Am J Pathol. 1981 Jul;104(1):77–89. [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Horoupian D. S., Terry R. D. Immunocytochemical comparison of neurofibrillary tangles in senile dementia of Alzheimer type, progressive supranuclear palsy, and postencephalitic parkinsonism. Ann Neurol. 1983 Feb;13(2):172–175. doi: 10.1002/ana.410130211. [DOI] [PubMed] [Google Scholar]