Abstract
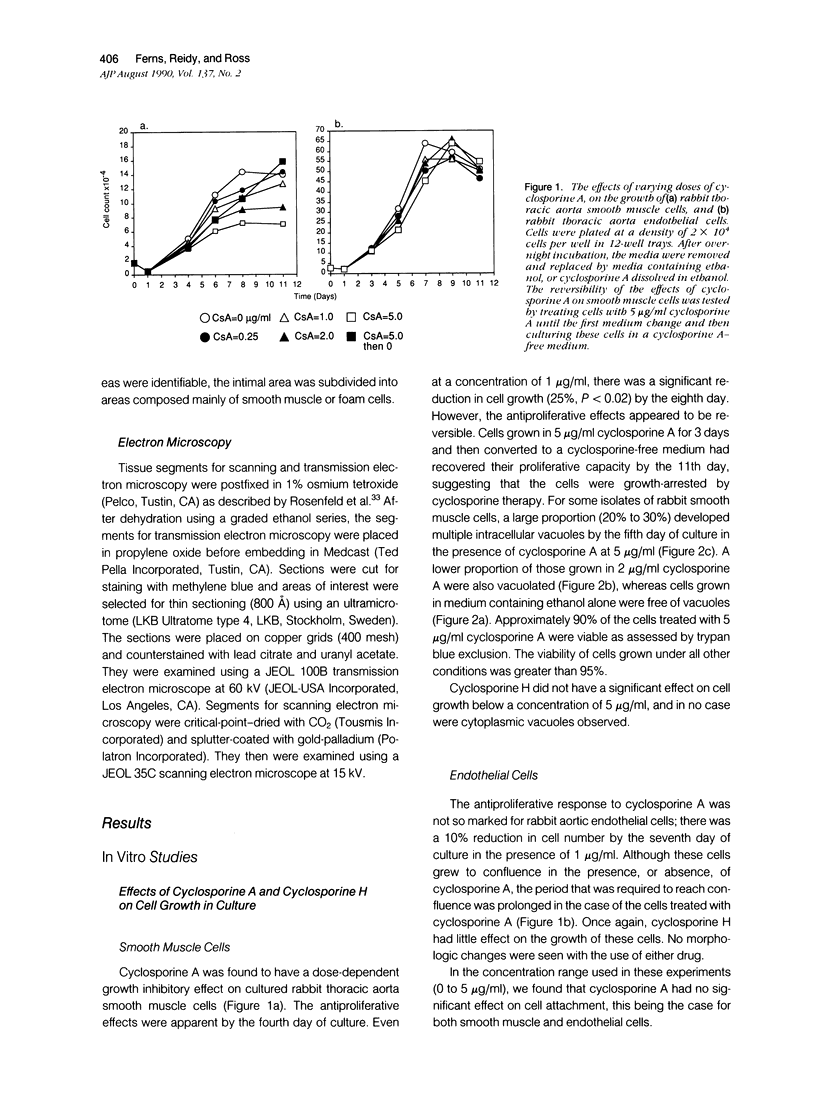
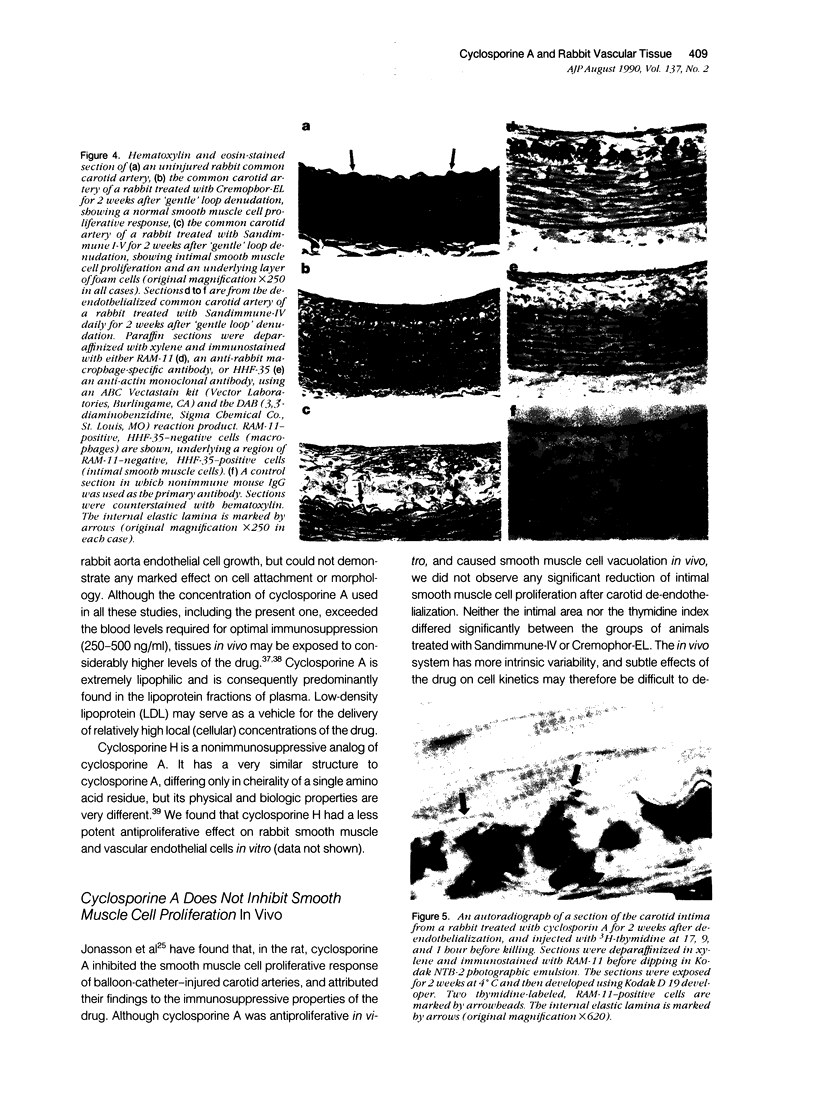
The authors have evaluated the in vivo effects of cyclosporine A on the de-endothelialized rabbit carotid artery. Vascular endothelium was gently removed from the common carotid artery of 10 New Zealand White rabbits that then were treated with therapeutic doses of cyclosporine A (15 mg/kg/day), or its vehicle (Cremophor-EL). Significant intimal proliferation was observed in all cases 2 weeks after de-endothelialization. Concomitant cyclosporine A therapy had no significant effect on intimal smooth muscle proliferation, but was associated with 1) intimal smooth muscle vacuolation, 2) an increase in total intimal thickening, because of the presence of numerous macrophage-derived foam cells, and 3) incorporation of 3H-thymidine by neo-intimal monocyte/macrophages. Mean plasma cholesterol levels were moderately increased in both groups. Although this may have contributed to foam cell formation in the cyclosporine-A-treated animals, it was not the sole determinant, as foam-cell-rich lesions were not observed in control animals. In vitro, cyclosporine A reduced the rate of proliferation of rabbit aortic smooth muscle and endothelial cells in a dose-dependent fashion, and induced smooth muscle cell vacuolation. These data suggest that cyclosporine A may contribute to the formation of graft-related atherosclerosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnarello A. G., Lewis L. A., McHenry M. C., Weinstein A. J., Naito H. K., McCullough A. J., Lederman R. J., Gavan T. L. Unusual serum lipoprotein abnormality induced by the vehicle of miconazole. N Engl J Med. 1977 Mar 3;296(9):497–499. doi: 10.1056/NEJM197703032960907. [DOI] [PubMed] [Google Scholar]
- Becker G. M., Gandolfi A. J., Nagle R. B. Effects of cyclosporin A on a kidney epithelial cell line (LLC-PK1). Res Commun Chem Pathol Pharmacol. 1987 May;56(2):277–280. [PubMed] [Google Scholar]
- Billingham M. E. Cardiac transplant atherosclerosis. Transplant Proc. 1987 Aug;19(4 Suppl 5):19–25. [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y., White D. J., Thiru S., Evans D. B., McMaster P., Dunn D. C., Craddock G. N., Pentlow B. D., Rolles K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978 Dec 23;2(8104-5):1323–1327. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A., Riederer I., Graf E., Speck B. Cyclosporine toxicity in rabbits. Lab Anim. 1986 Jul;20(3):213–220. doi: 10.1258/002367786780865692. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Harris K. P., Russell G. I., Parvin S. D., Veitch P. S., Walls J. Alterations in lipid and carbohydrate metabolism attributable to cyclosporin A in renal transplant recipients. Br Med J (Clin Res Ed) 1986 Jan 4;292(6512):16–16. doi: 10.1136/bmj.292.6512.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes A., Rowe P. A., Foster M. C., Steiger M. J., Morgan A. G. Cyclosporin toxicity and colitis. Lancet. 1988 Oct 22;2(8617):957–957. doi: 10.1016/s0140-6736(88)92618-9. [DOI] [PubMed] [Google Scholar]
- Inselmann G., Blank M., Baumann K. Cyclosporine A induced lipid peroxidation in microsomes and effect on active and passive glucose transport by brush border membrane vesicles of rat kidney. Res Commun Chem Pathol Pharmacol. 1988 Nov;62(2):207–220. [PubMed] [Google Scholar]
- Jevnikar A. M., Petric R., Holub B. J., Philbrick D. J., Clark W. F. Effect of cyclosporine on plasma lipids and modification with dietary fish oil. Transplantation. 1988 Nov;46(5):722–725. doi: 10.1097/00007890-198811000-00018. [DOI] [PubMed] [Google Scholar]
- Johnson D. E., Gao S. Z., Schroeder J. S., DeCampli W. M., Billingham M. E. The spectrum of coronary artery pathologic findings in human cardiac allografts. J Heart Transplant. 1989 Sep-Oct;8(5):349–359. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2303–2306. doi: 10.1073/pnas.85.7.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Kahn G. C., Shaw L. M., Kane M. D. Routine monitoring of cyclosporine in whole blood and in kidney tissue using high performance liquid chromatography. J Anal Toxicol. 1986 Jan-Feb;10(1):28–34. doi: 10.1093/jat/10.1.28. [DOI] [PubMed] [Google Scholar]
- Muskett A., Burton N. A., Eichwald E. J., Shelby J., Hendrickson M., Sullivan J. J. The effect of antiplatelet drugs on graft atherosclerosis in rat heterotopic cardiac allografts. Transplant Proc. 1987 Aug;19(4 Suppl 5):74–76. [PubMed] [Google Scholar]
- Nickoloff B. J., Fisher G. J., Mitra R. S., Voorhees J. J. Additive and synergistic antiproliferative effects of cyclosporin A and gamma interferon on cultured human keratinocytes. Am J Pathol. 1988 Apr;131(1):12–18. [PMC free article] [PubMed] [Google Scholar]
- Nitkin R. S., Hunt S. A., Schroeder J. S. Accelerated atherosclerosis in a cardiac transplant patient. J Am Coll Cardiol. 1985 Jul;6(1):243–245. doi: 10.1016/s0735-1097(85)80283-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Tsukada T., Gown A. M., Ross R. Fatty streak initiation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987 Jan-Feb;7(1):9–23. doi: 10.1161/01.atv.7.1.9. [DOI] [PubMed] [Google Scholar]
- Ryffel B., Donatsch P., Madörin M., Matter B. E., Rüttimann G., Schön H., Stoll R., Wilson J. Toxicological evaluation of cyclosporin A. Arch Toxicol. 1983 Jun;53(2):107–141. doi: 10.1007/BF00302721. [DOI] [PubMed] [Google Scholar]
- Sgoutas D., MacMahon W., Love A., Jerkunica I. Interaction of cyclosporin A with human lipoproteins. J Pharm Pharmacol. 1986 Aug;38(8):583–588. doi: 10.1111/j.2042-7158.1986.tb03085.x. [DOI] [PubMed] [Google Scholar]
- Shulman H., Striker G., Deeg H. J., Kennedy M., Storb R., Thomas E. D. Nephrotoxicity of cyclosporin A after allogeneic marrow transplantation: glomerular thromboses and tubular injury. N Engl J Med. 1981 Dec 3;305(23):1392–1395. doi: 10.1056/NEJM198112033052306. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Vaughan D. E., Rudd M. A., Mudge G. H., Kirshenbaum J., Young P., Alexander R. W., Loscalzo J. Frequency of hypercholesterolemia after cardiac transplantation. Am J Cardiol. 1988 Dec 1;62(17):1268–1272. doi: 10.1016/0002-9149(88)90272-x. [DOI] [PubMed] [Google Scholar]
- Sullivan B. A., Hak L. J., Finn W. F. Cyclosporine nephrotoxicity: studies in laboratory animals. Transplant Proc. 1985 Aug;17(4 Suppl 1):145–154. [PubMed] [Google Scholar]
- Tsukada T., Rosenfeld M., Ross R., Gown A. M. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986 Nov-Dec;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Uretsky B. F., Murali S., Reddy P. S., Rabin B., Lee A., Griffith B. P., Hardesty R. L., Trento A., Bahnson H. T. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- Whisler R. L., Lindsey J. A., Proctor K. V., Morisaki N., Cornwell D. G. Characteristics of cyclosporine induction of increased prostaglandin levels from human peripheral blood monocytes. Transplantation. 1984 Oct;38(4):377–381. doi: 10.1097/00007890-198410000-00012. [DOI] [PubMed] [Google Scholar]
- Whiting P. H., Thomson A. W., Simpson J. G. Cyclosporine: toxicity, metabolism, and drug interactions--implications from animal studies. Transplant Proc. 1985 Aug;17(4 Suppl 1):134–144. [PubMed] [Google Scholar]
- Zoja C., Furci L., Ghilardi F., Zilio P., Benigni A., Remuzzi G. Cyclosporin-induced endothelial cell injury. Lab Invest. 1986 Oct;55(4):455–462. [PubMed] [Google Scholar]