Abstract
Hypoxic–ischemic events sustained within the first year of life can result in developmental amnesia, a disorder characterized by markedly impaired episodic memory and relatively preserved semantic memory, in association with medial temporal pathology that appears to be restricted to the hippocampus. Here we compared children who had hypoxic–ischemic events before 1 year of age (early group, n = 6) with others who showed memory problems after suffering hypoxic–ischemic events between the ages of 6 and 14 years (late group, n = 5). Morphometric analyses of the whole brain revealed that, compared with age-matched controls, both groups had bilateral abnormalities in the hippocampus, putamen, and posterior thalamus, as well as in the right retrosplenial cortex. The two groups also showed similar reductions (≈40%) in hippocampal volumes. Neuropsychologically, the only significant differences between the two were on a few tests of immediate memory, where the early group surpassed the late group. The latter measures provided the only clear indication that very early injury can lead to greater functional sparing than injury acquired later in childhood, due perhaps to the greater plasticity of the infant brain. On measures of long-term memory, by contrast, the two groups had highly similar profiles, both showing roughly equivalent preservation of semantic memory combined with marked impairment in episodic memory. It thus appears that, if this selective memory disorder is a special syndrome related to the early occurrence of hypoxia-induced damage, then the effective age at injury for this syndrome extends from birth to puberty.
Bilateral hippocampal pathology incurred early in life as a result of a hypoxic–ischemic episode produces a relatively selective form of anterograde amnesia (1). We recently described a group of five children who had sustained such damage at birth or shortly after, whom we examined neuropsychologically between the ages of 8 and 19 years (2). In line with the initial report, all five children were found to be impaired in remembering the events of everyday life, an impairment so severe that each of them needed chronic supervision; yet they had acquired literacy skills and factual knowledge commensurate with their relatively intact intellectual abilities, which ranged between low average and high average. Their amnesic syndrome thus appears to be characterized by a disproportionate impairment in event, or episodic, memory as compared with fact, or semantic, memory (3). The hippocampal atrophy in these children was substantial, the volume of this structure ranging from 40% to 60% of normal on each side. Consistent with the hypoxic origin of the neuropathology, an etiology that can lead to frank motor signs up to and including cerebral palsy (4), voxel-based morphometry disclosed additional abnormalities in the putamen and ventral portions of the thalamus, although in our patients these other abnormalities were not accompanied by any obvious sensorimotor impairments (2). The syndrome that did result was labeled “developmental amnesia,” in recognition of the very early onset, within the medial temporal lobe, of the relatively selective bilateral hippocampal pathology that is the presumed cause of the pattern of impaired and spared memory abilities in these patients (5, 6).
The question remains, however, as to whether developmental amnesia (DA) differs in a major way from the amnesia that is produced by bilateral hippocampal damage sustained later in life, including later childhood. One possibility is that when such damage occurs in early infancy, at a time when neural plasticity is at its peak, one of the two major categories of cognitive memory function for which the mature hippocampus is commonly thought to be responsible, namely, semantic memory, can be taken over by extrahippocampal tissue; the other category, however, namely, episodic memory, apparently cannot be assumed by extrahippocampal tissue, or at least not so effectively, resulting in the relatively selective form of amnesia that has been observed. According to this view, the same damage incurred much later in childhood, when the brain has lost much of its capacity for major functional reorganization, could well result in a profile similar to the global amnesia commonly seen in adult-onset cases, in which semantic memory is thought to be impaired just as severely as episodic memory. Confirmation of this prediction would support the view that the hippocampus normally mediates both types of cognitive memory and that DA is indeed a special syndrome associated exclusively with very early damage to this structure. Alternatively, damage later in childhood could yield the same pattern and degree of selective mnemonic deficit as very early damage. Such an outcome would raise questions not only about the role of functional reorganization in DA but also, by implication, about the normal role of the hippocampus in semantic as compared with episodic memory.
We had the opportunity to examine this issue after having identified several patients who had suffered hypoxia-induced hippocampal pathology in middle to late childhood. We compared the memory abilities and the neuropathology of this new group with those of the children who had incurred hippocampal pathology in early infancy.
Methods
Participants. From our series of patients with memory problems associated with a history of hypoxic–ischemic damage sustained in childhood, 11 cases were selected to form two distinct age-at-injury groups. The early group consisted of six patients who had suffered hypoxic–ischemic episodes either perinatally or within the first 3 months of life. The late group consisted of five patients who had sustained such episodes between the ages of 6 and 14 years. Five of the patients in the early group were described in Gadian et al. (2); the sixth is a new patient who was referred to us after that report had been prepared. At the time of the initial referral, the patients ranged in age from 8 to 14 years. A brief description of each of the 11 patients is presented in Table 1.
Table 1. Details of patients in the early and late groups.
| Group | Etiology (initial event) | Sex | Age at injury, yr:mo | Age at test, yr:mo | Time from injury to test, yr:mo |
|---|---|---|---|---|---|
| Early | |||||
| E1 | Prematurity, respiratory arrest at 11 weeks | M | 0:3 | 12:4 | 12:1 |
| E2 | Extreme prematurity, hypoxia | M | 0 | 19:0 | 19:0 |
| E3 | Birth asphyxia, perinatal seizures | F | 0 | 12:10 | 12:10 |
| E4 | Hypoxia, perinatal seizures | M | 0 | 11:2 | 11:2 |
| E5 | Birth asphyxia, seizures at 7 yr | M | 0 | 11:1 | 11:1 |
| E6 | Prematurity, hypoxia | F | 0 | 11:0 | 11:0 |
| Late | |||||
| L1 | Cardiac arrest, hypoxia | F | 9:1 | 19:2 | 10:1 |
| L2 | Cardiac arrest, hypoxia (defibrillator implant) | M | 14:1 | 14:6 | 0:5 |
| L3 | Encephalitis, hypoxia | M | 6:0 | 14:11 | 8:11 |
| L4 | Meningitis, hypoxia | F | 12:5 | 14:7 | 2:2 |
| L5 | Herpes encephalitis, seizures (left temporal lobectomy) | M | 9:5 | 17:2 | 7:9 |
Worth noting here is a potentially important difference in the timing of the memory problems that brought the children in the two groups to our attention: The everyday memory difficulties of the early group either did not appear or were not recognized until about the time these children first entered school, whereas such difficulties in the children of the late group were apparent immediately after their hypoxic episode.
At the time of neuropsychological evaluation, the patients in the early group had a mean age of 13 years (range, 8–19), and the late group had a mean age of 16 years (range, 14–19), but the difference was not significant. The period from hypoxic episode to neuropsychological investigation in the early group ranged from 11 to 19 years, whereas it ranged from 5 months to 10 years in the late group.
Normal control data for those neuropsychological measures used in this study that have not been standardized for children were provided by groups of normal children with mean ages of 14 years or by published norms for 14-year-olds (see Tables 4 and 5).
Table 4. Delayed recall and Rivermead Behavioural Memory Test.
|
Early group
|
Late group
|
Early vs. late
|
Control group
|
||
|---|---|---|---|---|---|
| Test | X (SD) | X (SD) | t test | P value | X (SD) |
| WMS, delayed recall of stories, % | 4.6 (3.46) | 2.8 (4.04) | 1.02 | .33 | 32.4* (14.70) |
| WMS, delayed recall of paired associates (/10) | 5.0 (1.67) | 4.2 (1.64) | 1.27 | .24 | 9.7* (0.63) |
| WMS, delayed recall of geometric designs (/14) | 2.1 (1.32) | 3.6 (4.74) | -0.69 | .52 | 11.0* (2.32) |
| CAVLT-2, delayed recall (/16) | 3.3 (1.03) | 4.0 (2.92) | 0.53 | .61 | 10.8† (2.53) |
| CDLT, delayed recall (/9) | 2.2 (2.22) | 3.2 (3.70) | -0.45 | .67 | 7.80‡ (1.90) |
| REY, delayed recall (/36) | 1.7 (2.14) | 2.8 (3.85) | -0.03 | .98 | 26.2§ (5.40) |
| RBMT (/22) | 8.0 (2.53) | 9.8 (4.09) | -0.90 | .39 | (range, 20-22)¶ |
Scores are means (X) and SDs (or range where indicated). CDLT, Coughlan Design Learning Test; REY, Rey—Osterrieth Complex Figure; RBMT, Rivermead Behavioural Memory Test.
Normal controls (n = 36, mean age = 14 years, range 12-17 years).
Normal controls (n = 30, mean age = 14 years) (21).
Normal controls (n = 25, range 12-16 years) (25).
Normal controls (n = 180, age 14 years) (26).
Ref. 19.
Table 5. Immediate recall and intrusion errors.
|
Early group
|
Late group
|
Early vs. late
|
Control group
|
||
|---|---|---|---|---|---|
| Test | X (SD) | X (SD) | t test | P value | X (SD) |
| WMS, immediate recall of stories (%) | 29.5 (9.56) | 15.0 (4.64) | 3.25 | 0.013 | 40.9* (14.40) |
| WMS, trial 3 of paired associates, (/10) | 6.7 (1.51) | 4.6 (0.55) | 3.12 | 0.018 | 9.6* (0.73) |
| WMS, digit span, forward | 6.3 (0.52) | 5.2 (1.79) | 1.49 | 0.169 | 6.6* (1.21) |
| CAVLT-2, total intrusions | 19.5 (8.31) | 5.8 (3.11) | 3.46 | 0.007 | (range, 0-4)† |
Scores are means (X) and SDs (or range where indicated). (Digit Span forward is listed here because late, but not early, group differed significantly from control group. See Neuropsychology Results.)
Normal controls (n = 36, mean age = 14 years, range 12-17 years).
Normal controls (n = 30, mean age = 14 years) (21).
For morphometric analyses of the MRI scans, another group consisting of 16 normal children was used, with each patient group compared with its own controls (for early, n = 8, age range, 9–16 years; for late, n = 8, age range, 11–21 years).
The study was approved by the Great Ormond Street Hospital for Children (London)/Institute of Child Health Research Ethics Committee, and informed consent was obtained for each of the patients and control subjects.
Neuropathology. As indicated in Table 1, patient L2 had received a defibrillator implant and so could not participate in the MRI part of the study. All other patients, together with the 16 control subjects, were scanned on a 1.5-T Siemens Vision Scanner, with an inversion time-weighted 3D MPRAGE sequence (7) with the following parameters: repetition time 10 ms, echo time 4 ms, and inversion time 200 ms; flip angle 12°; matrix size, 256 = 256; field of view, 250 mm; partition thickness, 1.25 mm; 128 sagittal partitions in the third dimension; acquisition time, 8.5 min; no gap.
For voxel-based morphometry, which provides a measure of local gray-matter density, the 3D data sets were analyzed in spm99 (Wellcome Department of Imaging Neuroscience, London) according to the algorithm described in Ashburner and Friston (8). In brief, the images were normalized and then segmented into gray and white matter. The gray-matter images were then smoothed with a 4-mm isotropic Gaussian kernel, a size corresponding roughly to the cross-sectional dimension of the hippocampus, thereby rendering the analysis sensitive to differences at this spatial scale. The procedure was then repeated with an 8-mm kernel to increase the sensitivity of the analysis to the cross-sectional dimensions of the other structures of interest.
Similarities and differences between the early and late groups were tested with comparisons of the effects found in each group separately. Thus, conjunction analyses (9) were carried out to identify areas where the early and late groups, each compared with its own control group, had abnormalities in common. Similarly, contrasts were specified to test for an interaction between pathology and age of onset; these tested for the presence of significant differences between the early- and late-onset groups relative to their control groups. For both sets of analyses, proportional scaling to a grand mean of 100 was used to adjust for global gray-matter differences among the subjects, and contrast weights appropriate to each contrast were assigned to all four groups (the two patient groups and the two control groups). On the basis of the known neuropathology of hypoxic–ischemic injury (see, for example, refs. 10–13), we adopted the a priori hypothesis that the hippocampi, basal ganglia, and thalamus would show abnormalities. Other regions are reported only if they remain significant at P < 0.05 after whole-brain correction for multiple comparisons.
For the measurement of hippocampal volumes, the data sets were reformatted into 1-mm-thick contiguous slices in a tilted coronal plane perpendicular to the long axis of the hippocampus. Hippocampal cross-sectional areas were measured along the entire length of the hippocampus by using every third slice as described (2, 14). The volumes were calculated by summing the cross-sectional areas and multiplying by the distance between the measured slices. A correction was then made for intracranial volume, and the hippocampal volumes are presented here in this corrected form.
Neuropsychology. The patients were also assessed for intelligence [the age-appropriate test from the Wechsler Scales: Wechsler Intelligence Scale for Children (15) or Wechsler Adult Intelligence Scale (16)] and academic attainments, including reading, spelling, and reading comprehension [Wechsler Objective Reading Dimensions (WORD test; ref. 17)] and numerical abilities [Wechsler Objective Numerical Dimensions (WOND test; ref. 18)].
The patients were given a variety of memory tests, as follows: Memory for everyday events [the Rivermead Behavioural Memory Test (19)]; immediate and delayed memory [the Wechsler Memory Scale (WMS; ref. 20), with age corrections and adaptations for children]; auditory verbal learning [Children's Auditory Verbal Learning Test, revised (CAVLT-2; ref. 21)]; visual nonverbal learning [Coughlan Design Learning Test (22)] memory for a complex design [Rey–Osterrieth Complex Figure (23)]; and immediate or working memory [Digit Span, forward and backward (24)].
The testing of each patient was usually conducted in two sessions, with the WMS and Wechsler Intelligence Scales administered during the first session, and the remaining tests during the second session.
Results
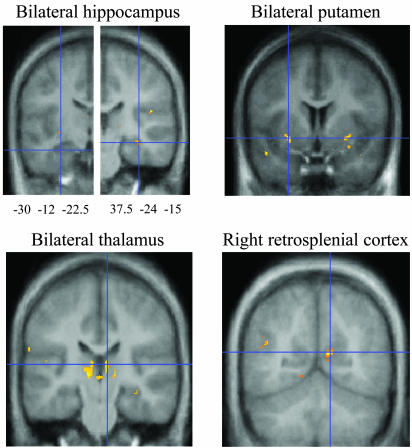
Neuropathology. Voxel-based morphometry. The conjunction analyses demonstrated that the early and late groups have several brain abnormalities in common, all of which are reflected in a reduction of gray-matter density relative to the density of those same regions in the control groups (Table 2 and Fig. 1). These abnormal areas include regions within the hippocampus, thalamus, and basal ganglia, each of these being abnormal bilaterally. An additional area of abnormality, this one unilateral, is located in the region of the right retrosplenial cortex. Although these regions are not necessarily the only ones that are structurally abnormal, no others reached the 0.05 level of significance after whole-brain correction for multiple comparisons.
Table 2. Reductions in gray-matter density common to both early and late groups, each compared with its own control group.
|
Coordinates
|
|||||||
|---|---|---|---|---|---|---|---|
| Region | Smoothing, mm | x | y | z | Corrected P value | Z score | Uncorrected P value |
| Left hippocampus | 4 | -30 | -12 | -22 | 3.89 | <0.001 | |
| Right hippocampus | 4 | 38 | -24 | -15 | 4.40 | <0.001 | |
| Right retrosplenial cortex | 4 | 12 | -60 | 14 | 0.002 | 5.85 | |
| Left putamen | 8 | -28 | 9 | -6 | 3.70 | <0.001 | |
| Right putamen | 8 | 32 | 3 | -8 | 3.63 | <0.001 | |
| Left caudate nucleus | 8 | -15 | 20 | -8 | 0.042 | 4.94 | |
| Right caudate nucleus | 8 | 14 | 20 | -6 | 3.38 | <0.001 | |
| Left thalamus | 8 | -12 | -21 | 4 | 3.87 | <0.001 | |
| Right thalamus | 8 | 8 | -22 | 9 | 4.03 | <0.001 | |
Unilateral conjunction analyses, MPRAGE (six early-onset patients, eight controls; four late-onset patients, eight controls). Corrected P values are included when they have reached significance at P < 0.05.
Fig. 1.
Results of voxel-based morphometry in patients versus controls. Conjunction analysis showing loci of reduced gray-matter density common to both early and late groups.
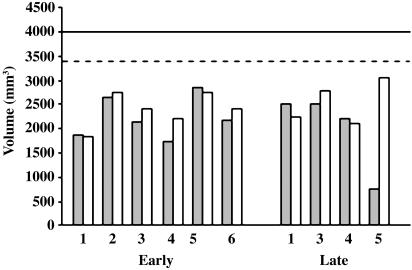
The interaction analyses failed to reveal any significant differences in the voxel-based morphometric measures between the two patient groups relative to their controls (P > 0.1 corrected). Hippocampal volumes. The hippocampal abnormalities were examined further, because these are presumed to be the major cause of the memory impairments produced by hypoxia-ischemia in our patients. Consistent with the voxel-based morphometric findings, the volumetric measurements indicated that each of the patients in the late group, like those in the early group (2), had substantial atrophy in both hippocampi, each one falling >2 SDs below the normal mean (Fig. 2). The average bilateral reduction in hippocampal volume, relative to the normal mean, was 43% (range, 30–54%) in the early group and 40% (range, 34–46%) in the late group.
Fig. 2.
Hippocampal volumes in early and late groups. Patient L2 with a defibrillator implant could not be scanned. Patient L5 had received a left temporal lobectomy. Filled bars, left hippocampus; open bars, right hippocampus; solid line, control mean; dashed line, 2 SDs below control mean.
As indicated in Table 1, patient L5 had received a left temporal lobectomy for relief of pharmaco-resistant epilepsy, accounting for the presence of only a hippocampal remnant in that hemisphere. Whether or not patient L5 is included in the comparison, the hippocampal volumes in the two groups do not differ reliably.
Neuropsychology. The mean verbal, performance, and full-scale IQ scores of both groups are in the average to low-average range (Table 3), and although the means of the early group exceed those of the late group by ≈7 points, the differences fall far short of significance. On the verbal subtests of information, vocabulary, and comprehension, presumed to be good indices of the ability to acquire semantic knowledge, again the differences between the two groups are not significant. Similarly, on the tests of academic achievement (WORD and WOND), on which the patients' scores are largely consistent with their IQ levels, none of the differences between the early and late groups are significant.
Table 3. Tests of intelligence and academic attainments.
|
Early group
|
Late group
|
Early vs. late
|
||
|---|---|---|---|---|
| Test | X (SD) | X (SD) | t test | P value |
| Wechsler Intelligence Scales | ||||
| Verbal IQ | 93.0 (11.87) | 86.4 (15.50) | 0.80 | 0.44 |
| Performance IQ | 88.5 (23.16) | 81.0 (18.45) | 0.58 | 0.57 |
| Full-scale IQ | 89.5 (18.79) | 82.2 (16.05) | 0.68 | 0.51 |
| Verbal IQ Subtests: Information, vocabulary, comprehension | 9.0 (1.74) | 7.0 (3.10) | 1.28 | 0.25 |
| WORD | ||||
| Basic reading | 96.8 (6.31) | 92.6 (18.50) | 0.53 | 0.61 |
| Spelling | 87.8 (7.96) | 87.8 (17.80) | 0.00 | 1.00 |
| Reading comprehension | 87.7 (11.76) | 85.0 (11.20) | 0.38 | 0.71 |
| WOND | ||||
| Math reasoning | 90.4 (8.93) | 81.5 (11.03) | 1.34 | 0.22 |
| Numerical operations | 98.4 (11.63) | 83.0 (6.56) | 2.06 | 0.08 |
Scores are means (X) and SDs. Means (±SDs) for normal subjects on the Wechsler Intelligence Scales, WORD, and WOND are 100 (±15). Means (±SDs) for normal subjects on the three verbal IQ subtests, which provide a measure of semantic memory, are 10 (±3).
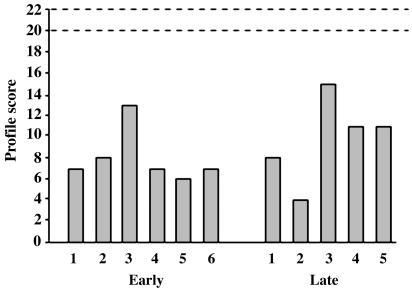
By contrast to their largely intact intelligence and academic attainments, both patient groups were severely impaired relative to controls on all of the tests considered to be measures of episodic memory (see Table 4). Yet on these tests, too, no significant differences occurred between the early and late groups. These measures included the Rivermead Behavioural Memory Test of everyday events (Fig. 3); delayed recall of the stories, paired associates, and designs of the WMS; and delayed recall of the revised Children's Auditory Verbal Learning Test, the Coughlan Design Learning Test, and the Rey–Osterrieth Complex Figure.
Fig. 3.
The Rivermead Behavioural Memory Test. The Standardized Profile Score is derived from the scores (0–2) on each of 11 subtests, including orientation to public and personal events, remembering a name, a belonging, a route, a story, an appointment, faces, line drawings of objects, and a message. The subtest of date, which is not included in the children's version of the Rivermead Behavioural Memory Test, was excluded from the profile score. Normal profile score ranges between 20 and 22.
Although the two patient groups did not differ significantly in age (see Participants), a weak trend toward an age difference occurred (P = 0.097), and, therefore, on the subtests of the WMS, which do not take age into account, we reanalyzed the results by using age as a covariate. The findings remained the same as before in each case.
A few memory tests did differentiate the two groups, but these were measures of immediate rather than delayed recall (see Table 5). Thus, on the WMS, the late group obtained lower scores than the early group on immediate recall of the stories (P = 0.013) and on trial 3 of the verbal paired associates (P = 0.018). On both of these measures and on forward digit span, the late group was also significantly impaired relative to the normal control group (P = 0.001, 0.001, and 0.032, respectively), whereas the early group was impaired relative to the controls only on trial 3 of the paired associates (P = 0.004).
The only other memory measure that distinguished reliably between the two patient groups was the total number of intrusion errors on lists 1 and 2 of CAVLT-2. Here, interestingly, the early group committed an average of >3 times as many intrusions as the late group. On the analogous nonverbal test (Coughlan Design Learning Test), the early group averaged more than twice as many total intrusions on the two different designs as the late group, but this difference did not reach significance.
Discussion
Our quantitative analyses of the neuropathology in the patients failed to reveal any differences between those who incurred their brain injury perinatally (the early group) and those who sustained their injury much later in childhood (the late group). Voxel-based morphometry disclosed areas of bilateral abnormality in the hippocampus, basal ganglia, and thalamus closely similar to (and, in the caudate nucleus region, extending) those identified in the early group (2). These loci of neuropathology are consistent with an etiology of hypoxia-ischemia (10–14). Also newly revealed in this study is the unilateral neuropathology in the right retrosplenial cortex.
The relationship of the extrahippocampal abnormalities to the patients' memory impairments is unknown, although a significant contribution from at least the right retrosplenial neuropathology seems highly probable, given the evidence that this area is commonly activated in neuroimaging studies of memory in normal subjects (e.g., refs. 27–29). Nevertheless, the memory disorder is presumably due primarily to the marked bilateral hippocampal atrophy, an abnormality confirmed with both voxel-based morphometry and direct volumetric measurements. The only evidence of a motor impairment that might be attributable to the putamen damage was a mild clumsiness, which later recovered, in some members of the early group. Interestingly, however, despite the later onset of their damage, and the sudden onset of their everyday memory problems (see Participants), none of the members of the late group showed any such motor signs.
The neuropsychological profiles of the two patient groups, like the neuropathological profiles, were highly similar. First, both groups showed roughly equivalent preservation of semantic memory ability. Thus, no significant differences between the early and late groups were found on any of the assessments of intelligence or academic attainments, both groups having obtained mean scores in the low-average to average range. This similarity of the two groups in the relative preservation of semantic learning ability despite marked impairment in episodic memory seems remarkable, considering that the early group had acquired all their semantic information in the 13 years, on average, that had elapsed between their perinatal hippocampal injury and their neuropsychological evaluation (see Table 1). The late group, by contrast, had had an average of 10 years of normal memory development and presumably retained much of the semantic knowledge they had acquired during that period. In addition, however, unlike most reported patients with adult-onset amnesia, the late group must have also added substantially to their long-term memory store during the average period of 6 years that elapsed between the time of their later-onset hippocampal pathology and the time of testing; otherwise, they would have failed to attain or maintain an age-corrected level of intelligence and academic attainment in the low-average to average range.
Second, both groups showed equally severe impairments in episodic memory. On these tests, too, no differences were observed between the two groups in any of the measures, verbal or nonverbal, of either everyday memory or delayed recall, where the retention intervals ranged from ≈15 to 90 min. Apparently, unlike the storage and retrieval of semantic information, the storage and retrieval of episodic memories is critically dependent on the hippocampus and perhaps secondarily dependent on one or more of the other structures that were found to be compromised in both groups of patients.
Only in tests of immediate or working memory, where the retention intervals were on the order of 15–30 s, did significant group differences emerge. Here, although the early group was impaired on one of the measures, the late group often showed the greater impairment. The tests of immediate memory thus provide the only indication in this study that perinatal injury can lead to greater functional sparing than very similar injury acquired later in childhood, due perhaps to the greater plasticity of the infant brain. It is unclear whether this superiority in the early group, which may have come at the expense of an abnormal number of intrusion errors, reflects a better working memory ability or an increased sensory-processing capacity. In either case, the long-term episodic and semantic memory abilities of the two groups do not appear to differ. The findings thus lead to the tentative conclusion that, if DA is indeed a special syndrome related to the early occurrence of hypoxia-induced hippocampal damage, then the effective age at injury for this syndrome to appear extends from birth to puberty, and possibly beyond.
As already noted, the patients with DA have a seemingly selective pattern of medial temporal neuropathology. Thus, whereas bilateral hippocampal atrophy is clearly visible on their MRI scans, no obvious pathology is found on visual inspection of the underlying parahippocampal region, consisting of the entorhinal, perirhinal, and parahippocampal cortices. This seemingly isolated medial temporal damage, supported by the results of the quantitative MRI techniques that have been applied thus far (refs. 1 and 2; D. Schoppik, personal communication), is consistent with the notion that whereas the DA patients' episodic memory impairment is due mainly to their hippocampal pathology, their relatively preserved semantic memory could be related to the integrity of the underlying cortices (5, 6). There are, however, alternative possibilities, namely, that the limited form of amnesia in our young patients is due instead either to partial sparing of the hippocampal formation or to a degree of functional reorganization and compensation after very early injury that is not possible after damage acquired later in life.
The decision between these alternatives will require direct comparison with patients who have had still later onsets of the same amount and apparent selectivity of medial temporal pathology as those described here. Recently, at least three patients have been reported whose anterograde amnesia was incurred in adulthood and whose damage appears to be restricted to the hippocampus (30–32). Despite the presence of a severe episodic memory impairment in each of these patients, each has also shown at least some degree of new semantic learning, although the extent of the newly acquired information is minimal compared with the level acquired by our patients with DA. Moreover, several other case studies of patients with adult-onset injury seemingly limited to the hippocampus have failed to uncover any evidence of preserved semantic memory ability (33–35). Whether this difference in semantic memory outcome is due in fact to the different ages at injury is still uncertain, however, inasmuch as undetermined differences could still exist between the adult- and childhood-onset cases in the extent of the neuropathology. Indeed, further quantitative study of the neuropathology in the hippocampus and parahippocampal region in both the childhood and adult forms of amnesia is needed to help decide among the possible explanations of the specific memory profile seen in DA.
Acknowledgments
We are indebted to the patients and their families and the control subjects for their cooperation with this study. We thank Elizabeth Isaacs and Anna Adlam for their help with statistical analyses. Support for C.H.S. was provided by a Medical Research Council Ph.D. Studentship. Additional support was provided by the Wellcome Trust and the National Institute of Mental Health Intramural Research Program/National Institutes of Health/Department of Health and Human Services.
Abbreviations: WMS, Wechsler Memory Scale; DA, developmental amnesia; WORD, Wechsler Objective Reading Dimensions; WOND, Wechsler Objective Numerical Dimensions; CAVLT-2, Children's Auditory Verbal Learning Test, revised.
References
- 1.Vargha-Khadem, F., Gadian, D. G., Watkins, K. E., Connelly, A., Van Paesschen, W. & Mishkin, M. (1997) Science 277, 376–380. [DOI] [PubMed] [Google Scholar]
- 2.Gadian, D. G., Aicardi, J., Watkins, K. E., Porter, D. A., Mishkin, M. & Vargha-Khadem, F. (2000) Brain 123, 499–507. [DOI] [PubMed] [Google Scholar]
- 3.Tulving, E. & Markowitsch, H. J. (1998) Hippocampus 8, 198–204. [DOI] [PubMed] [Google Scholar]
- 4.Perlman, J. M., Broyles, R. S. & Rogers, C. G. (1997) Pediatr. Neurol. 4, 322–326. [DOI] [PubMed] [Google Scholar]
- 5.Mishkin, M., Suzuki, W., Gadian, D. & Vargha-Khadem, F. (1997) Philos. Trans. R. Soc. London B 352, 1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishkin, M., Vargha-Khadem, F. & Gadian, D. G. (1998) Hippocampus 8, 212–216. [DOI] [PubMed] [Google Scholar]
- 7.Mugler, J. P. & Brookeman, J. R. (1990) Magn. Reson. Med. 15, 152–157. [DOI] [PubMed] [Google Scholar]
- 8.Ashburner, J. & Friston, K. J. (2000) NeuroImage 11, 805–821. [DOI] [PubMed] [Google Scholar]
- 9.Salmond, C. H., Ashburner, J., Vargha-Khadem, F., Gadian, D. G. & Friston, K. J. (2000) Hum. Brain Mapp. 11, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutherford, M. A., Pennock, J. M. Schwieso, J. E., Cowan, F. M. & Dubowitz, L. M. (1994) Dev. Med. Child. Neurol. 36, 813–825. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford, M. A., Pennock, J. M., Schwieso, J. E., Cowan, F. M. & Dubowitz, L. M. (1995) Neuropediatrics 26, 183–191. [DOI] [PubMed] [Google Scholar]
- 12.Barkovich, A. J. & Hallam, D. (1997)Ment. Retard. Dev. Disabil. Res. Rev. 3, 28–41. [Google Scholar]
- 13.Mercuri, E., Guzzetta, A., Haataja, L., Cowan, F., Rutherford, M., Counsell S., Papadimitriou, M., Cioni, G. & Dubowitz, L. (1999) Neuropediatrics 30, 83–90. [DOI] [PubMed] [Google Scholar]
- 14.Van Paesschen, W., Revesz, T., Duncan, J. S., King, M. D. & Connelly, A. (1997) Ann. Neurol. 42, 756–766. [DOI] [PubMed] [Google Scholar]
- 15.Bracken, B. A., ed. (1992) Wechsler Intelligence Scale for Children (Psychological Corp., Sidcup, Kent, U.K.), 3rd Ed.
- 16.Wechsler, D. (1981) Manual for the Wechsler Adult Intelligence Scale (Psychological Corp., Sidcup, Kent, U.K.), Revised U.K. Ed.
- 17.Rust, J., Golombok, S. & Trickey, G. (1993) Wechsler Objective Reading Dimensions (Psychological Corp., Sidcup, Kent, U.K.).
- 18.Rust, J. (1996) Wechsler Objective Numerical Dimensions Manual (Psychological Corp., London).
- 19.Wilson, B., Cockburn, J. & Baddeley, A. (1985) Rivermead Behavioural Memory Test (Thames Valley Test, Reading, U.K.).
- 20.Wechsler, D. (1945) J. Psychol. 19, 87–95. [Google Scholar]
- 21.Talley, J. L. (1993) Children's Auditory Verbal Learning Test (Psychological Assessment Resources, Odessa, FL).
- 22.Coughlan, J. & Hollows, S. E. (1985) Adult Memory and Information Processing Battery (St. James Hospital, Leeds, U.K.).
- 23.Rey, A. (1964) L'Examen Clinique en Psychologie (Presses Universitaire de France, Paris).
- 24.Isaacs, E. & Vargha-Khadem, F. (1989) Br. J. Dev. Psychol. 7, 377–380. [Google Scholar]
- 25.Wood, S. (1998) Ph.D. thesis (Univ. of London, London).
- 26.Kolb, B. & Wishaw, I. Q. (1990) Fundamentals of Human Neuropsychology (Freeman, New York), 3rd Ed.
- 27.Desgranges, B., Baron, J., Lalevée, C., Giffard, B., Viader, F., de la Sayette, V. & Eustache, F. (2002) Brain 125, 1116–1124. [DOI] [PubMed] [Google Scholar]
- 28.Shah, N. J., Marshall, J. C., Zafiris, O., Schwab, A., Zilles, K., Markowitsch, H. J. & Fink, G. R. (2001) Brain 124, 804–815. [DOI] [PubMed] [Google Scholar]
- 29.Maguire, E., Vargha-Khadem, F. & Mishkin, M. (2001) Brain 124, 1156–1170. [DOI] [PubMed] [Google Scholar]
- 30.Kitchener, E. G., Hodges, J. R. & McCarthy, R. (1998) Brain 121, 1313–1327. [DOI] [PubMed] [Google Scholar]
- 31.Holdstock, J. S., Mayes, A. R., Cezayirli, E., Isaac, C. L., Aggleton, J. P. & Roberts, N. (2000) Neuropsychologia 38, 410–425. [DOI] [PubMed] [Google Scholar]
- 32.Verfaellie, M., Koseff, P. & Alexander, M. P. (2000) Neuropsychologia 38, 484–492. [DOI] [PubMed] [Google Scholar]
- 33.Kartsounis, L. D., Rudge, P. & Stevens, J. M. (1995) J. Neurol. Neurosurg. Psychiatry 59, 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed, J. M. & Squire, L. R. (1998) J. Neurosci. 18, 3943–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manns, J. R., Hopkins, R. O. & Squire, L. R. (2003) Neuron 38, 127–133. [DOI] [PubMed] [Google Scholar]