Abstract
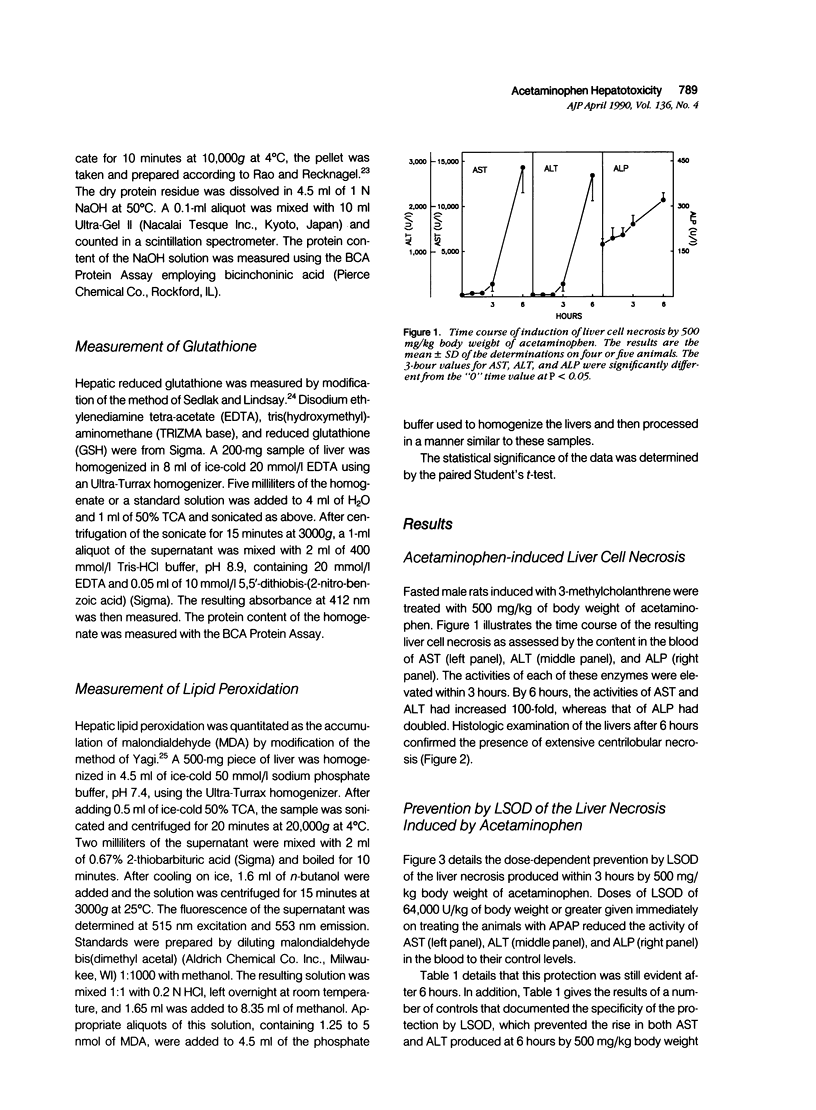
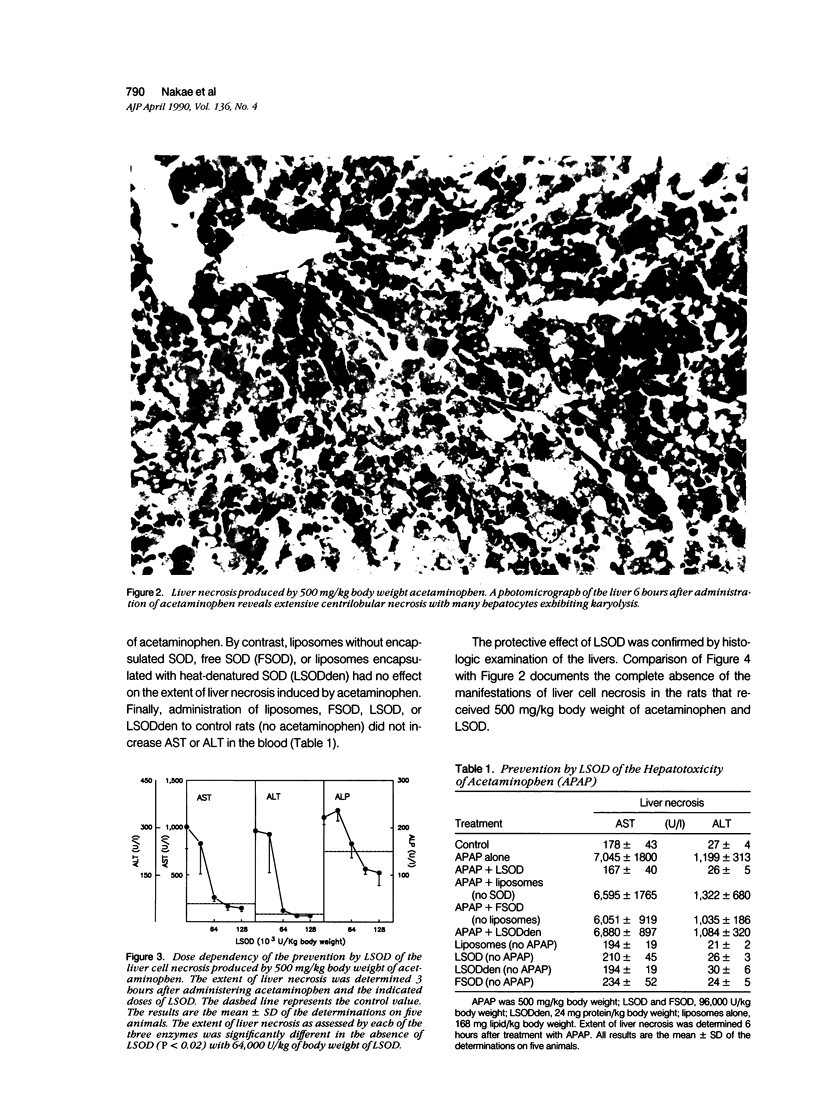
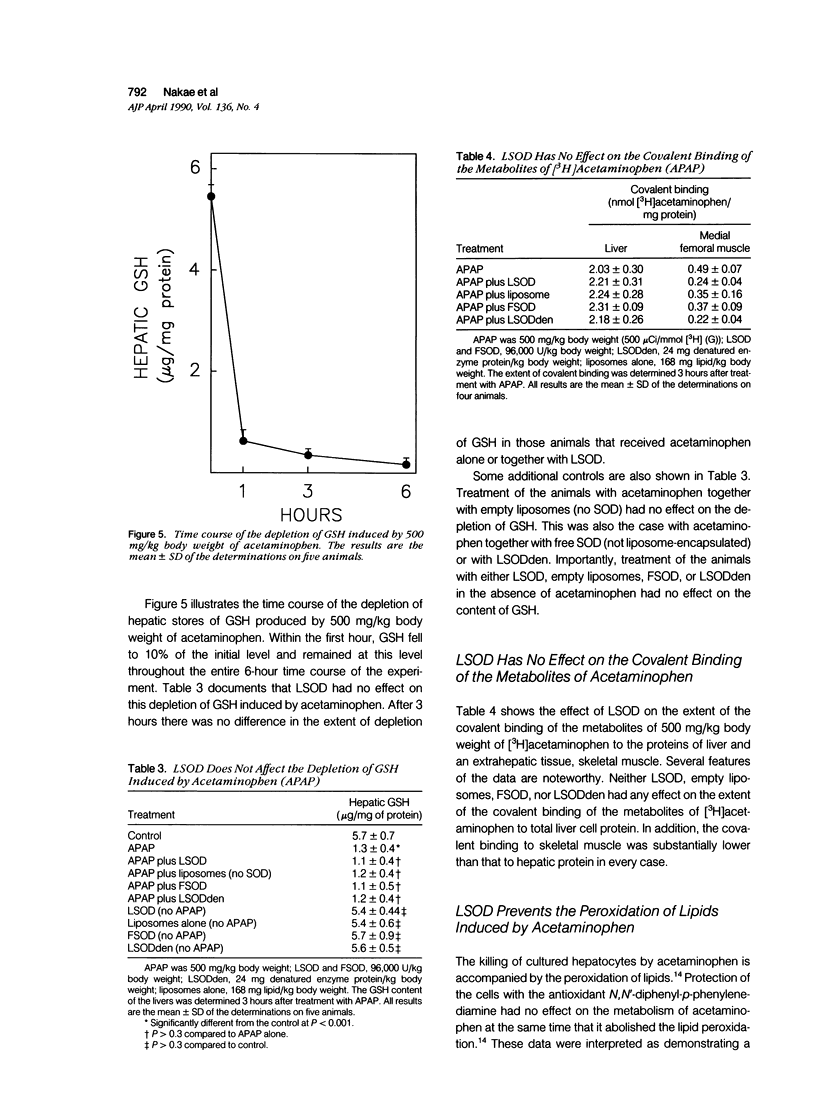
Liposome-encapsulated human recombinant superoxide dismutase (LSOD) protected male rats that were pretreated with 3-methylcholanthrene from the liver necrosis produced by acetaminophen. By contrast, SOD-free liposomes, free SOD, or heat-denatured LSOD had no protective effect. Liposome-encapsulated SOD did not simply delay the onset of liver necrosis. A second dose of LSOD at 12 hours prevented the necrosis of the liver as assessed 24 hours after treatment with 500 mg/kg body weight of acetaminophen. Liposome-encapsulated human recombinant superoxide dismutase did not alter the metabolism of acetaminophen as assessed by either the rate or extent of the depletion of hepatic stores of glutathione or by the extent of the covalent binding of the metabolites of [3H]acetaminophen to total liver cell proteins. Evidence of the peroxidation of lipids in the accumulation of malondialdehyde in the livers was detected within 3 hours of the administration of acetaminophen and before the appearance of liver necrosis. Liposome-encapsulated human recombinant superoxide dismutase prevented the accumulation of malondialdehyde in parallel with the prevention of liver necrosis. Finally, LSOD also prevented the potentiation by 1,3-bis(2-chloroethyl)-1-nitrosourea of the hepatotoxicity of acetaminophen. These data document the participation of superoxide anions in the hepatotoxicity of acetaminophen in intact rats.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Jewell S. A., Thor H., Orrenius S. Regulation of intracellular calcium compartmentation: studies with isolated hepatocytes and t-butyl hydroperoxide. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6842–6846. doi: 10.1073/pnas.79.22.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers G. N., Jr, McComb R. B. Measurement of total alkaline phosphatase activity in human serum. Clin Chem. 1975 Dec;21(13):1988–1995. [PubMed] [Google Scholar]
- Dahlin D. C., Miwa G. T., Lu A. Y., Nelson S. D. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson R. J., Casini A., Gilfor D., Serroni A., Farber J. L. Oxygen-mediated cell injury in the killing of cultured hepatocytes by acetaminophen. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1129–1137. doi: 10.1016/0006-291x(85)90303-1. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973 Oct;187(1):195–202. [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Zampaglione N., Gillette J. R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Thorgeirsson S. S., Potter W. Z., Hashimoto M., Mitchell J. R. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology. 1974;12(4-5):251–271. doi: 10.1159/000136547. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Miccadei S., Nakae D., Farber J. L. Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem Biophys Res Commun. 1987 Dec 31;149(3):889–896. doi: 10.1016/0006-291x(87)90491-8. [DOI] [PubMed] [Google Scholar]
- Kyle M. E., Nakae D., Serroni A., Farber J. L. 1,3-(2-Chloroethyl)-1-nitrosourea potentiates the toxicity of acetaminophen both in the phenobarbital-induced rat and in hepatocytes cultured from such animals. Mol Pharmacol. 1988 Oct;34(4):584–589. [PubMed] [Google Scholar]
- Michelson A. M., Puget K. Cell penetration by exogenous superoxide dismutase. Acta Physiol Scand Suppl. 1980;492:67–80. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Nakae D., Oakes J. W., Farber J. L. Potentiation in the intact rat of the hepatotoxicity of acetaminophen by 1,3-bis(2-chloroethyl)-1-nitrosourea. Arch Biochem Biophys. 1988 Dec;267(2):651–659. doi: 10.1016/0003-9861(88)90073-2. [DOI] [PubMed] [Google Scholar]
- Potter W. Z., Davis D. C., Mitchell J. R., Jollow D. J., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther. 1973 Oct;187(1):203–210. [PubMed] [Google Scholar]
- Potter W. Z., Thorgeirsson S. S., Jollow D. J., Mitchell J. R. Acetaminophen-induced hepatic necrosis. V. Correlation of hepatic necrosis, covalent binding and glutathione depletion in hamsters. Pharmacology. 1974;12(3):129–143. doi: 10.1159/000136531. [DOI] [PubMed] [Google Scholar]
- Rao K. S., Recknagel R. O. Early incorporation of carbon-labeled carbon tetrachloride into rat liver particulate lipids and proteins. Exp Mol Pathol. 1969 Apr;10(2):219–228. doi: 10.1016/0014-4800(69)90041-0. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985 Jan 10;260(1):86–92. [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Wendel A., Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981 Sep 15;30(18):2513–2520. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S., Konz K. H. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979 Jul 1;28(13):2051–2055. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]
- Wendel A., Jaeschke H., Gloger M. Drug-induced lipid peroxidation in mice--II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem Pharmacol. 1982 Nov 15;31(22):3601–3605. doi: 10.1016/0006-2952(82)90582-2. [DOI] [PubMed] [Google Scholar]
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976 Apr;15(2):212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]




