Abstract
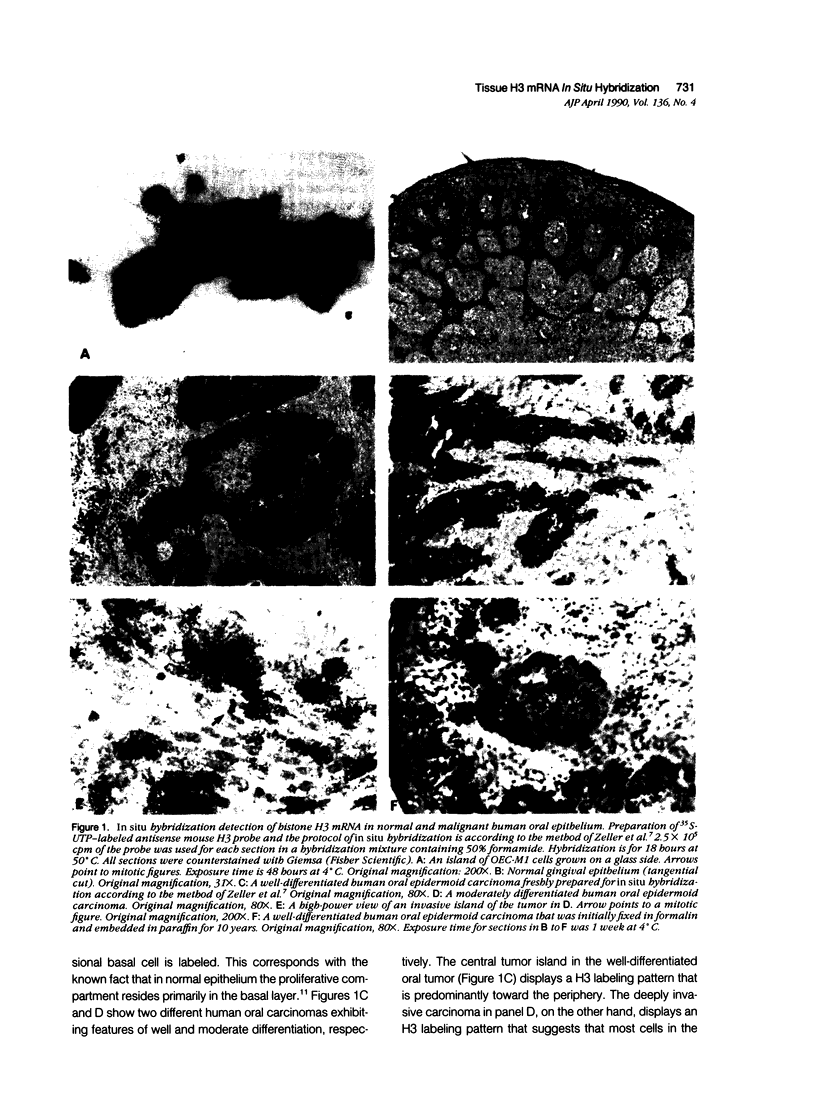
A general method applicable for the determination of any mammalian tissue's proliferative pattern is described. This method determines the cellular mRNA level of a proliferation-dependent gene, histone H3, by in situ hybridization. The cell-cycle S-phase-specific expression of this highly conserved ubiquitous cellular gene, and the lack of it in resting cells, permits the unambiguous identification of cycling cells in any tissues, normal or diseased. This method can be conveniently coupled with routine biopsy and could be streamlined for a central laboratory with results obtainable in 2 days. Furthermore, this procedure works successfully on formalin-fixed paraffin-embedded sections, thus allowing retrospective studies of biopsies or autopsy materials.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. C., Chou M. Y., Chow P., Matossian K., McBride J., Tao C. A., Gallagher G. T., Wong D. T. Detection of transforming growth factor-alpha messenger RNA in normal and chemically transformed hamster oral epithelium by in situ hybridization. Cancer Res. 1989 Dec 1;49(23):6700–6707. [PubMed] [Google Scholar]
- Hoffman R. M., Connors K. M., Meerson-Monosov A. Z., Herrera H., Price J. H. A general native-state method for determination of proliferation capacity of human normal and tumor tissues in vitro. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2013–2017. doi: 10.1073/pnas.86.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H., Villnave C. A., Stein J. L., Stein G. S. Intracellular distribution of histone mRNAs in human fibroblasts studied by in situ hybridization. Proc Natl Acad Sci U S A. 1988 Jan;85(2):463–467. doi: 10.1073/pnas.85.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDivitt R. W., Stone K. R., Craig R. B., Palmer J. O., Meyer J. S., Bauer W. C. A proposed classification of breast cancer based on kinetic information: derived from a comparison of risk factors in 168 primary operable breast cancers. Cancer. 1986 Jan 15;57(2):269–276. doi: 10.1002/1097-0142(19860115)57:2<269::aid-cncr2820570214>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- McDivitt R. W., Stone K. R., Meyer J. S. A method for dissociation of viable human breast cancer cells that produces flow cytometric kinetic information similar to that obtained by thymidine labeling. Cancer Res. 1984 Jun;44(6):2628–2633. [PubMed] [Google Scholar]
- Meyer J. S., Connor R. E. In vitro labeling of solid tissues with tritiated thymidine for autoradiographic detection of S-phase nuclei. Stain Technol. 1977 Jul;52(4):185–195. doi: 10.3109/10520297709116774. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Chiu I. M., Pan C. J., Cohn R. H., Kedes L. H., Marzluff W. F. Isolation of two clusters of mouse histone genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4078–4082. doi: 10.1073/pnas.78.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R., Bloch K. D., Williams B. S., Arceci R. J., Seidman C. E. Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev. 1987 Sep;1(7):693–698. doi: 10.1101/gad.1.7.693. [DOI] [PubMed] [Google Scholar]