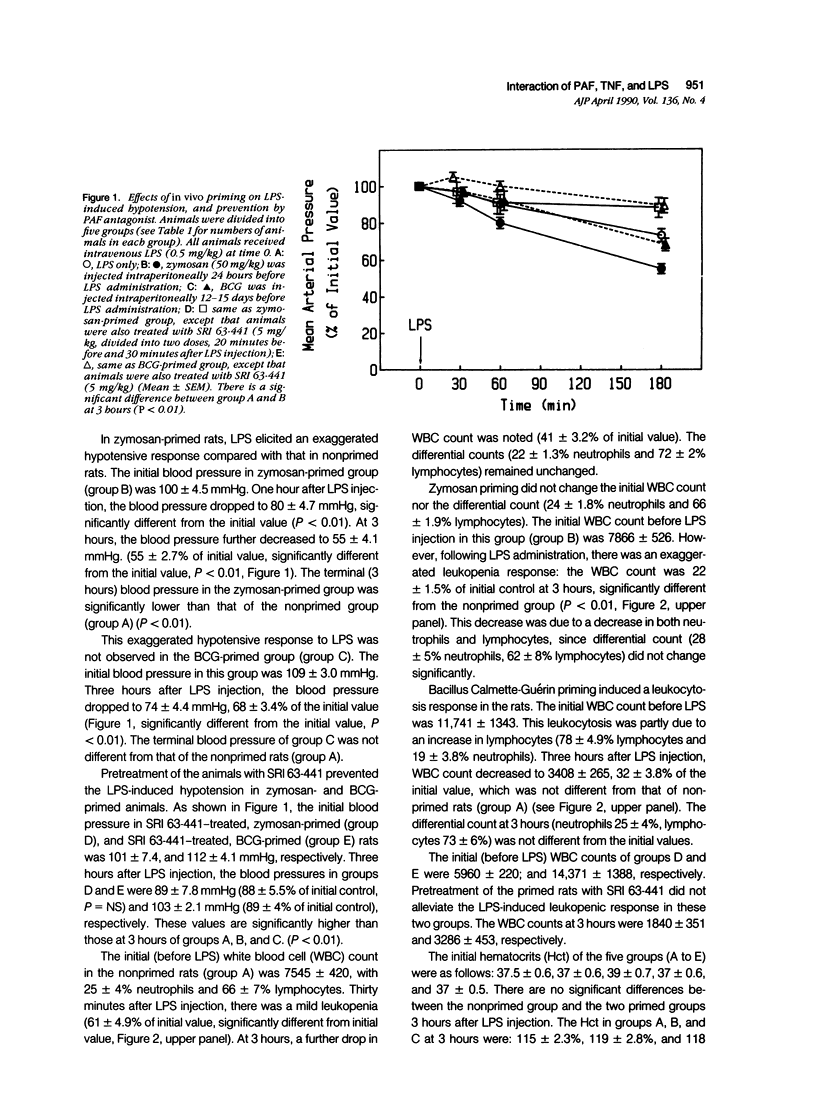
Abstract
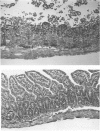
Exogenously administered tumor necrosis factor-alpha (TNF) and bacterial endotoxin (LPS) induce shock and tissue injury. Here, the authors studied the effect of endogenous TNF on LPS-induced hypotension and tissue injury and investigated the role of PAF in these responses. Rats were primed with intraperitoneal injection of zymosan 24 hours before, or Bacillus Calmette-Guérin (BCG) 12 to 15 days before intravenous injection of low dose (0.5 mg/kg) LPS. It was found that nonprimed animals showed mild hypotension and moderate leukopenia in response to LPS. In contrast, zymosanprimed rats developed shock and marked leukopenia, and more severe bowel injury than nonprimed rats. The authors then showed that, following LPS injection, zymosan-primed animals had higher TNF and platelet-activating factor (PAF) levels than nonprimed rats. Pretreatment of the animal with PAF antagonist, SRI 63-441, markedly ameliorated the hypotension and tissue injury. Interestingly, BCG-primed rats did not show aggravation of LPS-induced hypotension. Only TNF (but not PAF) level in these animals was increased. Thus, it appears that TNF release alone, without a sufficient increase in PAF, is incapable of causing severe hypotension. However, most of the BCG-primed animals showed tissue injury, which could be prevented by pretreatment with PAF antagonist. The authors discuss the possible mechanisms of this discrepancy between systemic and local responses in BCG-primed animals.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Bessin P., Bonnet J., Apffel D., Soulard C., Desgroux L., Pelas I., Benveniste J. Acute circulatory collapse caused by platelet-activating factor (PAF-acether) in dogs. Eur J Pharmacol. 1983 Jan 21;86(3-4):403–413. doi: 10.1016/0014-2999(83)90190-5. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Salvidio G., Baglioni C. Tumor necrosis factor/cachectin stimulates peritoneal macrophages, polymorphonuclear neutrophils, and vascular endothelial cells to synthesize and release platelet-activating factor. J Exp Med. 1987 Nov 1;166(5):1390–1404. doi: 10.1084/jem.166.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. W., Feddersen C. O., Henson P. M., Voelkel N. F. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987 May;79(5):1498–1509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Robbins J. C., Choy B. M., Chang M. N., Shen T. Y. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun. 1985 Mar 29;127(3):799–808. doi: 10.1016/s0006-291x(85)80014-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gonzalez-Crussi F., Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am J Pathol. 1983 Jul;112(1):127–135. [PMC free article] [PubMed] [Google Scholar]
- Grandel K. E., Farr R. S., Wanderer A. A., Eisenstadt T. C., Wasserman S. I. Association of platelet-activating factor with primary acquired cold urticaria. N Engl J Med. 1985 Aug 15;313(7):405–409. doi: 10.1056/NEJM198508153130702. [DOI] [PubMed] [Google Scholar]
- Halonen M., Palmer J. D., Lohman I. C., McManus L. M., Pinckard R. N. Respiratory and circulatory alterations induced by acetyl glyceryl ether phosphorylcholine, a mediator of IgE anaphylaxis in the rabbit. Am Rev Respir Dis. 1980 Dec;122(6):915–924. doi: 10.1164/arrd.1980.122.6.915. [DOI] [PubMed] [Google Scholar]
- Hsueh W., González-Crussi F., Arroyave J. L. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987 Nov;1(5):403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldawer L. L., Gelin J., Scherstén T., Lundholm K. G. Circulating interleukin 1 and tumor necrosis factor during inflammation. Am J Physiol. 1987 Dec;253(6 Pt 2):R922–R928. doi: 10.1152/ajpregu.1987.253.6.R922. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafforini D. M., McIntyre T. M., Carter M. E., Prescott S. M. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J Biol Chem. 1987 Mar 25;262(9):4215–4222. [PubMed] [Google Scholar]
- Sun X. M., Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988 May;81(5):1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Urban J. L., Schreiber H. Selection of macrophage-resistant progressor tumor variants by the normal host. Requirement for concomitant T cell-mediated immunity. J Exp Med. 1983 Feb 1;157(2):642–656. doi: 10.1084/jem.157.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]