Abstract
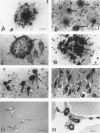
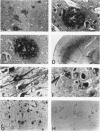
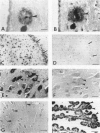
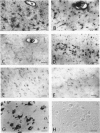
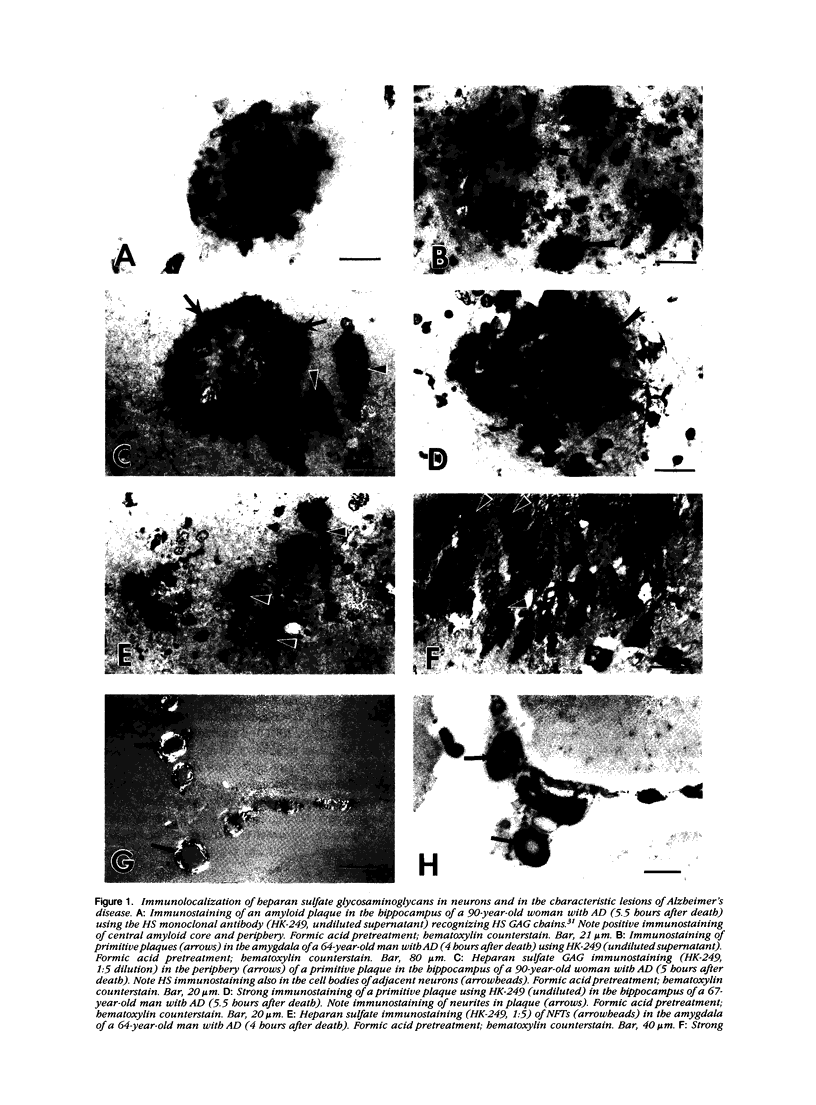
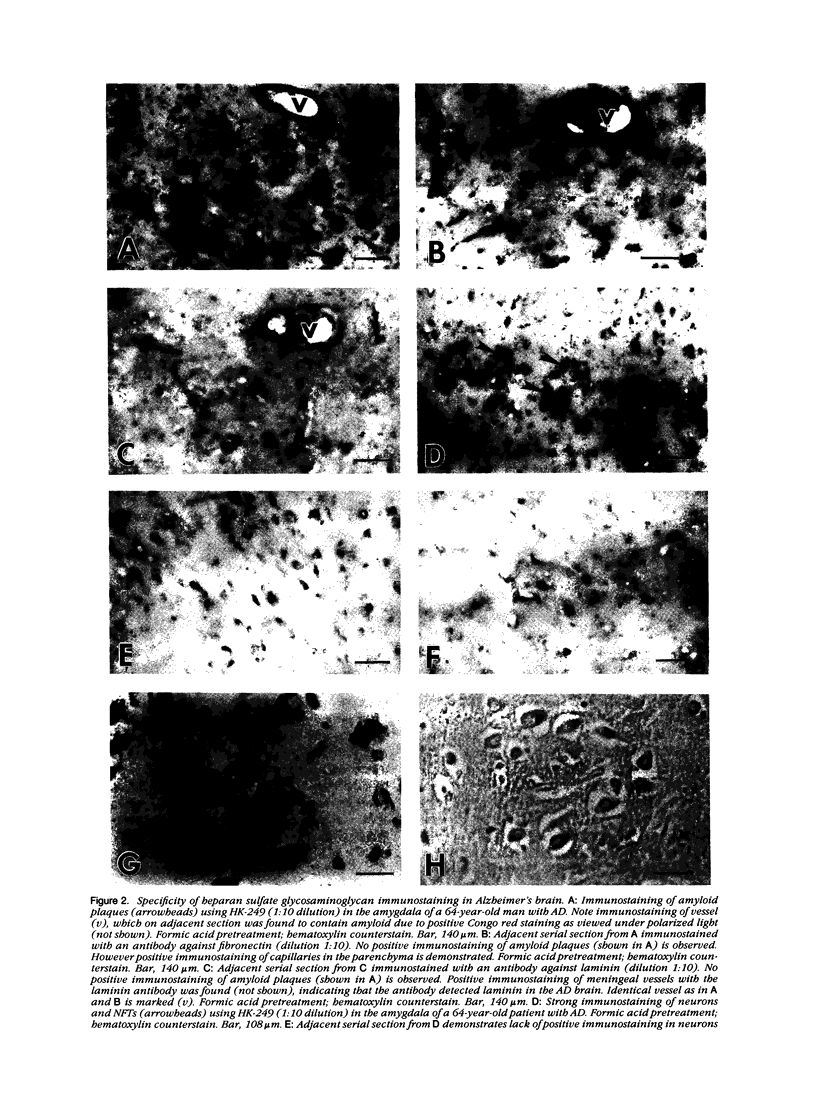
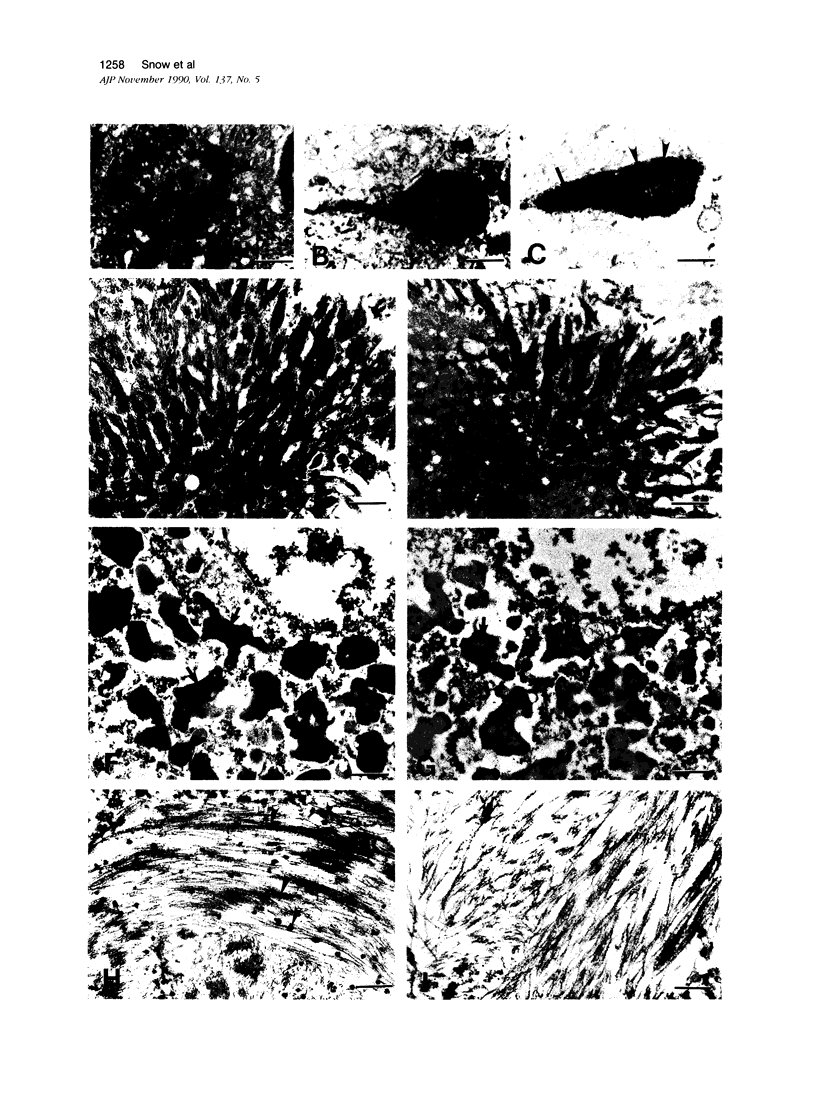
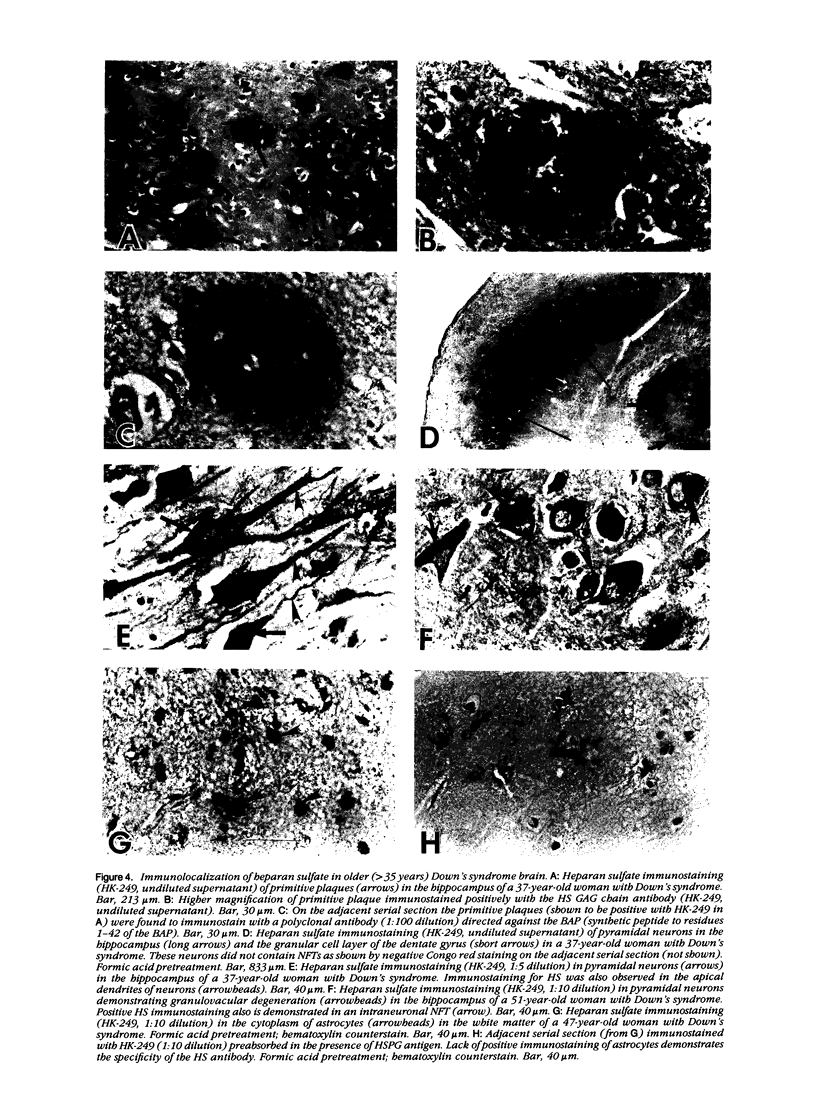
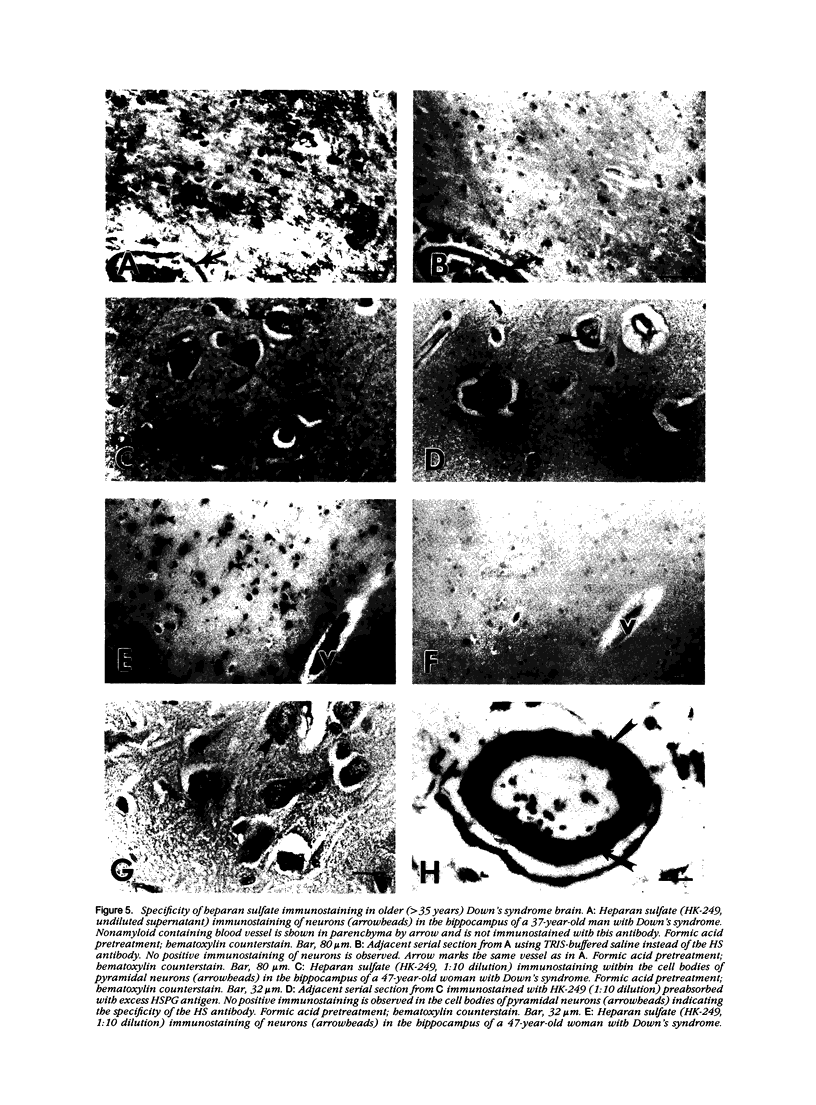
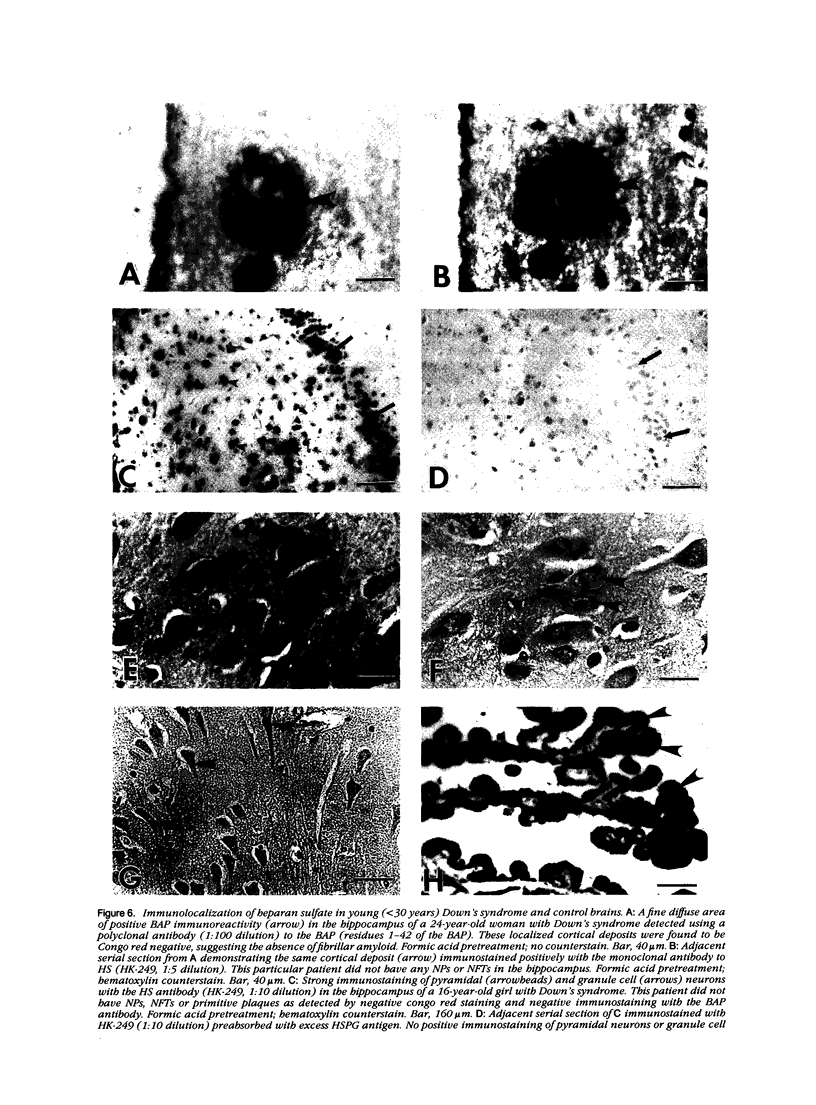
A monoclonal antibody (HK-249) that recognizes a glucosamine sulfate alpha 1----4 glucuronic acid-containing determinant in heparan sulfate (HS) chains of a basement membrane-derived heparan sulfate proteoglycan identified and immunolocalized HS specifically to the amyloid deposits in neuritic plaques (NPs), congophilic angiopathy (CA), as well as in neurofibrillary tangles (NFTs) and non-tangle-bearing neurons in the brains of Alzheimer's and Down's syndrome (DS) patients. Ultrastructural immunohistochemistry demonstrated that HS within neurons of Alzheimer's disease (AD) brain was localized to lipofuscin granules, an aging pigment previously shown also to contain beta-amyloid protein (BAP). Heparan sulfate also was localized to neurite-containing, nonfibrillar 'primitive' plaques that also demonstrated positive BAP immunoreactivity in both AD and DS brains. Antibodies to laminin, fibronectin, and a chondroitin sulfate proteoglycan failed to show positive immunostaining of the HS-containing sites described above. Analysis of DS patients at different ages revealed that HS accumulated within neurons of the hippocampus and amygdala as early as 1 day after birth. Young age-matched controls did not demonstrate similar positive HS immunoreactivity in neurons, whereas positive immunostaining for HS was observed in other regions thought to normally contain HS. The earliest deposition of BAP was first observed as 'amorphous' or 'diffuse' cortical deposits in DS brain in patients aged 18 and 24 years before the accumulation of fibrillar amyloid (observed in DS patients who are 35 years and older). These cortical deposits also contained positive HS immunoreactivity, implying that HS accumulation in conjunction with the BAP is an early event that ultimately may contribute to the early age-related accumulation (ie, as early as 35 years of age in DS) of NPs, NFTs, and/or CA. Furthermore the colocalization of HS and BAP in a number of specific locales in AD and DS brain indicates a possible interaction between these two macromolecules that may be important in lesion development in these two diseases.
Full text
PDF






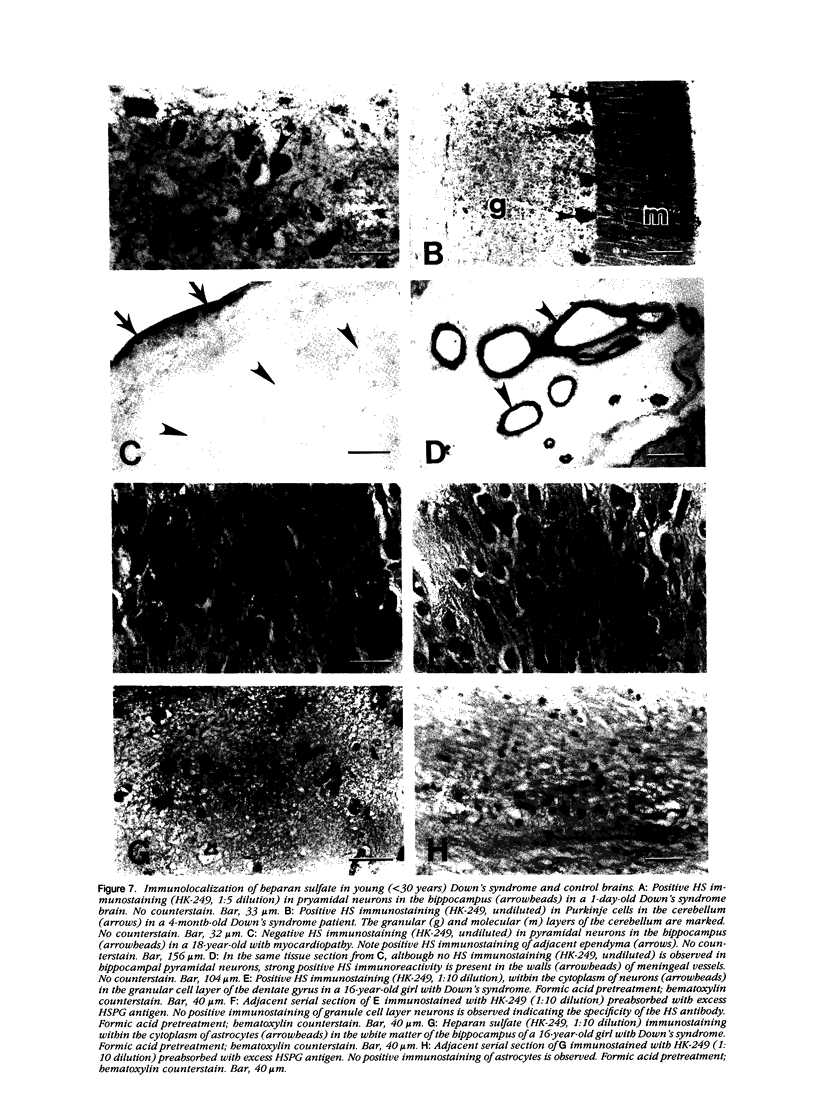




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Avila J. L., Convit J. Inhibition of leucocytic lysosomal enzymes by glycosaminoglycans in vitro. Biochem J. 1975 Oct;152(1):57–64. doi: 10.1042/bj1520057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Convit J. Physicochemical characteristics of the glycosaminoglycan-lysosomal enzyme interaction in vitro. A model of control of leucocytic lysosomal activity. Biochem J. 1976 Nov 15;160(2):129–136. doi: 10.1042/bj1600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Bancher C., Grundke-Iqbal I., Iqbal K., Kim K. S., Wisniewski H. M. Immunoreactivity of neuronal lipofuscin with monoclonal antibodies to the amyloid beta-protein. Neurobiol Aging. 1989 Mar-Apr;10(2):125–132. doi: 10.1016/0197-4580(89)90021-3. [DOI] [PubMed] [Google Scholar]
- Coleman P. D., Flood D. G. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987 Nov-Dec;8(6):521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Dorfman A., Matalon R. The Hurler and Hunter syndromes. Am J Med. 1969 Nov;47(5):691–707. doi: 10.1016/0002-9343(69)90164-8. [DOI] [PubMed] [Google Scholar]
- Dowson J. H. Neuronal lipofuscin accumulation in ageing and alzheimer dementia: a pathogenic mechanism? Br J Psychiatry. 1982 Feb;140:142–148. doi: 10.1192/bjp.140.2.142. [DOI] [PubMed] [Google Scholar]
- Eshhar Z., Blatt C., Bergman Y., Heimovich J. Induction of secretion of IgM from cells of the B cell line 38c-13 by somatic cell hybridization. J Immunol. 1979 Jun;122(6):2430–2434. [PubMed] [Google Scholar]
- Farrell D. H., Cunningham D. D. Glycosaminoglycans on fibroblasts accelerate thrombin inhibition by protease nexin-1. Biochem J. 1987 Jul 15;245(2):543–550. doi: 10.1042/bj2450543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W., Quaranta V., Eanes E. D. The amyloid deposits in Alzheimer's disease: their nature and pathogenesis. Appl Pathol. 1984;2(6):357–369. [PubMed] [Google Scholar]
- Gorenstein C., Roberts V. J., Bundman M. C. Redistribution of lipofuscin in aged neurons induced by colchicine. Mech Ageing Dev. 1988 Jan;42(1):63–73. doi: 10.1016/0047-6374(88)90063-2. [DOI] [PubMed] [Google Scholar]
- Goyal V. K. Lipofuscin pigment accumulation in human brain during aging. Exp Gerontol. 1982;17(6):481–487. doi: 10.1016/s0531-5565(82)80010-7. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., George L., Tung Y. C., Kim K. S., Wisniewski H. M. Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2853–2857. doi: 10.1073/pnas.86.8.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh M. C., Probst A., Ulrich J., Kahn J., Anderton B. H. Alzheimer neurofibrillary tangles contain phosphorylated and hidden neurofilament epitopes. J Neurol Neurosurg Psychiatry. 1986 Nov;49(11):1213–1220. doi: 10.1136/jnnp.49.11.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A., Dembitzer H. M., Kurland L. T., Zimmerman H. M. The fine structure of some intraganglionic alterations. Neurofibrillary tangles, granulovacuolar bodies and "rod-like" structures as seen in Guam amyotrophic lateral sclerosis and parkinsonism-dementia complex. J Neuropathol Exp Neurol. 1968 Apr;27(2):167–182. [PubMed] [Google Scholar]
- Kato M., Koike Y., Suzuki S., Kimata K. Basement membrane proteoglycan in various tissues: characterization using monoclonal antibodies to the Engelbreth-Holm-Swarm mouse tumor low density heparan sulfate proteoglycan. J Cell Biol. 1988 Jun;106(6):2203–2210. doi: 10.1083/jcb.106.6.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T., Ogomori K., Tateishi J., Prusiner S. B. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest. 1987 Aug;57(2):230–236. [PubMed] [Google Scholar]
- Koike F., Kunishita T., Nakayama H., Tabira T. Immunohistochemical study of Alzheimer's disease using antibodies to synthetic amyloid and fibronectin. J Neurol Sci. 1988 May;85(1):9–15. doi: 10.1016/0022-510x(88)90031-7. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Joachim C. L., Selkoe D. J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986 Jun;83(11):4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F., Williams R. S. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989 Aug;46(8):849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- Lark M. W., Yeo T. K., Mar H., Lara S., Hellström I., Hellström K. E., Wight T. N. Arterial chondroitin sulfate proteoglycan: localization with a monoclonal antibody. J Histochem Cytochem. 1988 Oct;36(10):1211–1221. doi: 10.1177/36.10.3047228. [DOI] [PubMed] [Google Scholar]
- Mann D. M. Alzheimer's disease and Down's syndrome. Histopathology. 1988 Aug;13(2):125–137. doi: 10.1111/j.1365-2559.1988.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Brown A., Prinja D., Davies C. A., Landon M., Masters C. L., Beyreuthers K. An analysis of the morphology of senile plaques in Down's syndrome patients of different ages using immunocytochemical and lectin histochemical techniques. Neuropathol Appl Neurobiol. 1989 Jul-Aug;15(4):317–329. doi: 10.1111/j.1365-2990.1989.tb01232.x. [DOI] [PubMed] [Google Scholar]
- Mann D. M. The pathogenesis and progression of the pathological changes of Alzheimer's disease. Ann Med. 1989;21(2):133–136. doi: 10.3109/07853898909149200. [DOI] [PubMed] [Google Scholar]
- Mann D. M. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988 May;43(2):99–136. doi: 10.1016/0047-6374(88)90041-3. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O. Lipoprotein pigments--their relationship to ageing in the human nervous system. I. The lipofuscin content of nerve cells. Brain. 1974 Sep;97(3):481–488. doi: 10.1093/brain/97.1.481. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Marcyniuk B. Changes in nerve cells of the nucleus basalis of Meynert in Alzheimer's disease and their relationship to ageing and to the accumulation of lipofuscin pigment. Mech Ageing Dev. 1984 Apr-May;25(1-2):189–204. doi: 10.1016/0047-6374(84)90140-4. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Yates P. O., Marcyniuk B. Relationship between pigment accumulation and age in Alzheimer's disease and Down syndrome. Acta Neuropathol. 1984;63(1):72–77. doi: 10.1007/BF00688473. [DOI] [PubMed] [Google Scholar]
- Mar H., Tsukada T., Gown A. M., Wight T. N., Baskin D. G. Correlative light and electron microscopic immunocytochemistry on the same section with colloidal gold. J Histochem Cytochem. 1987 Apr;35(4):419–425. doi: 10.1177/35.4.3546488. [DOI] [PubMed] [Google Scholar]
- Mar H., Wight T. N. Colloidal gold immunostaining on deplasticized ultra-thin sections. J Histochem Cytochem. 1988 Nov;36(11):1387–1395. doi: 10.1177/36.11.2844888. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin W. D., Kay C. M., Narindrasorasak S., Kisilevsky R. Circular-dichroism studies on two murine serum amyloid A proteins. Biochem J. 1988 Dec 15;256(3):775–783. doi: 10.1042/bj2560775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Muir H. The structure and metabolism of mucopolysaccharides (glycosaminoglycans) and the problem of the mucopolysaccharidoses. Am J Med. 1969 Nov;47(5):673–690. doi: 10.1016/0002-9343(69)90163-6. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Fritz L. C., Schenk D. B., Lieberburg I., Johnson-Wood K. L., Beattie E. C., Ward P. J., Blacher R. W., Dovey H. F., Sinha S. The secreted form of the Alzheimer's amyloid precursor protein with the Kunitz domain is protease nexin-II. Nature. 1989 Sep 14;341(6238):144–147. doi: 10.1038/341144a0. [DOI] [PubMed] [Google Scholar]
- Pallis C. A., Duckett S., Pearse A. G. Diffuse lipofuscinosis of the central nervous system. Neurology. 1967 Apr;17(4):381–394. doi: 10.1212/wnl.17.4.381. [DOI] [PubMed] [Google Scholar]
- Palmert M. R., Podlisny M. B., Witker D. S., Oltersdorf T., Younkin L. H., Selkoe D. J., Younkin S. G. Antisera to an amino-terminal peptide detect the amyloid protein precursor of Alzheimer's disease and recognize senile plaques. Biochem Biophys Res Commun. 1988 Oct 14;156(1):432–437. doi: 10.1016/s0006-291x(88)80859-3. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt D. E., Geula C., Mesulam M. M. Protease nexin I immunostaining in Alzheimer's disease. Ann Neurol. 1989 Nov;26(5):628–634. doi: 10.1002/ana.410260507. [DOI] [PubMed] [Google Scholar]
- Rozemuller J. M., Eikelenboom P., Stam F. C., Beyreuther K., Masters C. L. A4 protein in Alzheimer's disease: primary and secondary cellular events in extracellular amyloid deposition. J Neuropathol Exp Neurol. 1989 Nov;48(6):674–691. doi: 10.1097/00005072-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Schroeder R., LaCorbiere M., Saitoh T., Cole G. Amyloid beta protein precursor is possibly a heparan sulfate proteoglycan core protein. Science. 1988 Jul 8;241(4862):223–226. doi: 10.1126/science.2968652. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Bergman B. L., Bajpai A., Hersh R. T., Rodriguez H., Jones B. N., Barreda C., Watts S., Baker J. B. Protease nexin. Properties and a modified purification procedure. J Biol Chem. 1985 Jun 10;260(11):7029–7034. [PubMed] [Google Scholar]
- Selkoe D. J. Altered structural proteins in plaques and tangles: what do they tell us about the biology of Alzheimer's disease? Neurobiol Aging. 1986 Nov-Dec;7(6):425–432. doi: 10.1016/0197-4580(86)90055-2. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Lara S., Nochlin D., Wight T. N. Cationic dyes reveal proteoglycans structurally integrated within the characteristic lesions of Alzheimer's disease. Acta Neuropathol. 1989;78(2):113–123. doi: 10.1007/BF00688198. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Mar H., Nochlin D., Kimata K., Kato M., Suzuki S., Hassell J., Wight T. N. The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988 Dec;133(3):456–463. [PMC free article] [PubMed] [Google Scholar]
- Snow A. D., Wight T. N. Proteoglycans in the pathogenesis of Alzheimer's disease and other amyloidoses. Neurobiol Aging. 1989 Sep-Oct;10(5):481–497. doi: 10.1016/0197-4580(89)90108-5. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Willmer J. P., Kisilevsky R. Sulfated glycosaminoglycans in Alzheimer's disease. Hum Pathol. 1987 May;18(5):506–510. doi: 10.1016/s0046-8177(87)80036-9. [DOI] [PubMed] [Google Scholar]
- Sommer J., Gloor S. M., Rovelli G. F., Hofsteenge J., Nick H., Meier R., Monard D. cDNA sequence coding for a rat glia-derived nexin and its homology to members of the serpin superfamily. Biochemistry. 1987 Oct 6;26(20):6407–6410. doi: 10.1021/bi00394a016. [DOI] [PubMed] [Google Scholar]
- Stern R. A., Otvos L., Jr, Trojanowski J. Q., Lee V. M. Monoclonal antibodies to a synthetic peptide homologous with the first 28 amino acids of Alzheimer's disease beta-protein recognize amyloid and diverse glial and neuronal cell types in the central nervous system. Am J Pathol. 1989 May;134(5):973–978. [PMC free article] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubold R. D. Studies on chemical nature of lipofusion (age pigment) isolated from normal human brain. Lipids. 1975 Jul;10(7):383–390. doi: 10.1007/BF02532441. [DOI] [PubMed] [Google Scholar]
- Tsuchida M., Miura T., Aibara K. Lipofuscin and lipofuscin-like substances. Chem Phys Lipids. 1987 Jul-Sep;44(2-4):297–325. doi: 10.1016/0009-3084(87)90055-7. [DOI] [PubMed] [Google Scholar]
- Van Nostrand W. E., Wagner S. L., Suzuki M., Choi B. H., Farrow J. S., Geddes J. W., Cotman C. W., Cunningham D. D. Protease nexin-II, a potent antichymotrypsin, shows identity to amyloid beta-protein precursor. Nature. 1989 Oct 12;341(6242):546–549. doi: 10.1038/341546a0. [DOI] [PubMed] [Google Scholar]
- Wallace A., Rovelli G., Hofsteenge J., Stone S. R. Effect of heparin on the glia-derived-nexin-thrombin interaction. Biochem J. 1989 Jan 1;257(1):191–196. doi: 10.1042/bj2570191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. D. A quantitative study of lipofuscin accumulation with age in normals and individuals with Down's syndrome, phenylketonuria, progeria and transneuronal atrophy. J Comp Neurol. 1979 Jul 1;186(1):109–116. doi: 10.1002/cne.901860108. [DOI] [PubMed] [Google Scholar]
- Wisniewski H. M., Bancher C., Barcikowska M., Wen G. Y., Currie J. Spectrum of morphological appearance of amyloid deposits in Alzheimer's disease. Acta Neuropathol. 1989;78(4):337–347. doi: 10.1007/BF00688170. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H., Hirai S., Morimatsu M., Shoji M., Harigaya Y. Diffuse type of senile plaques in the brains of Alzheimer-type dementia. Acta Neuropathol. 1988;77(2):113–119. doi: 10.1007/BF00687420. [DOI] [PubMed] [Google Scholar]