Abstract
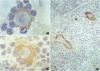
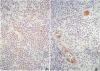
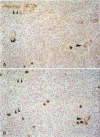
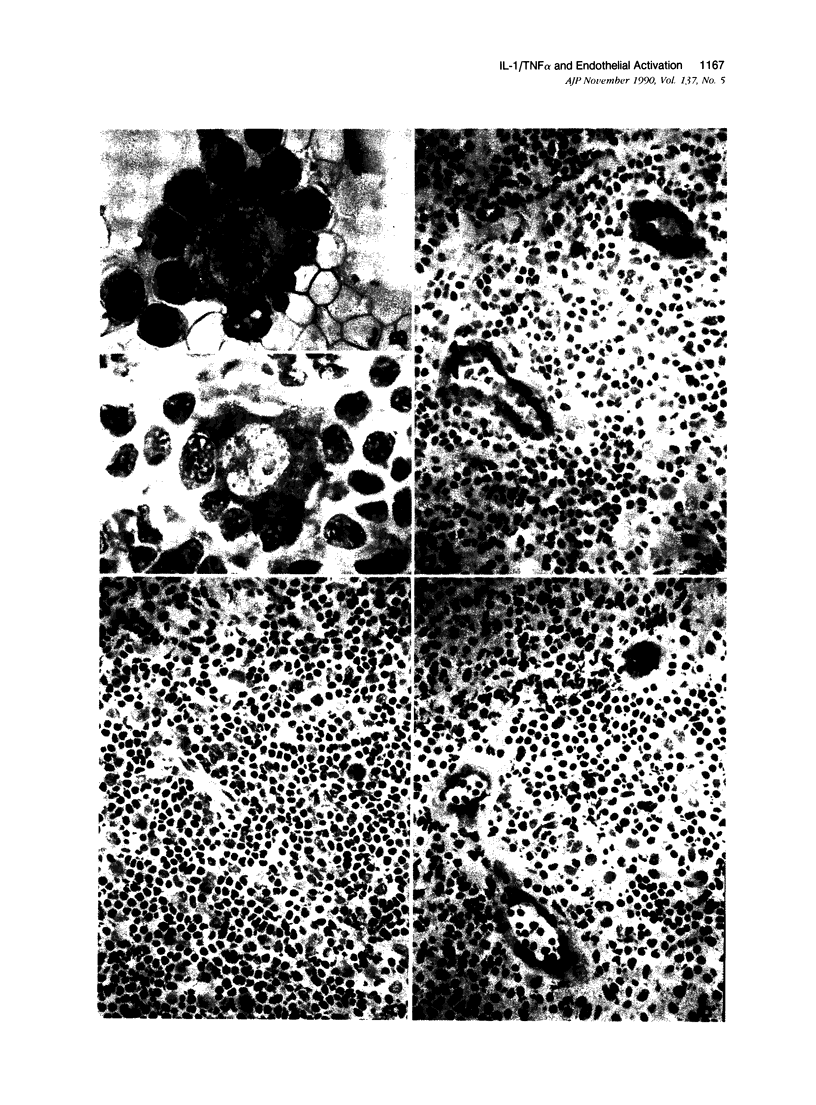
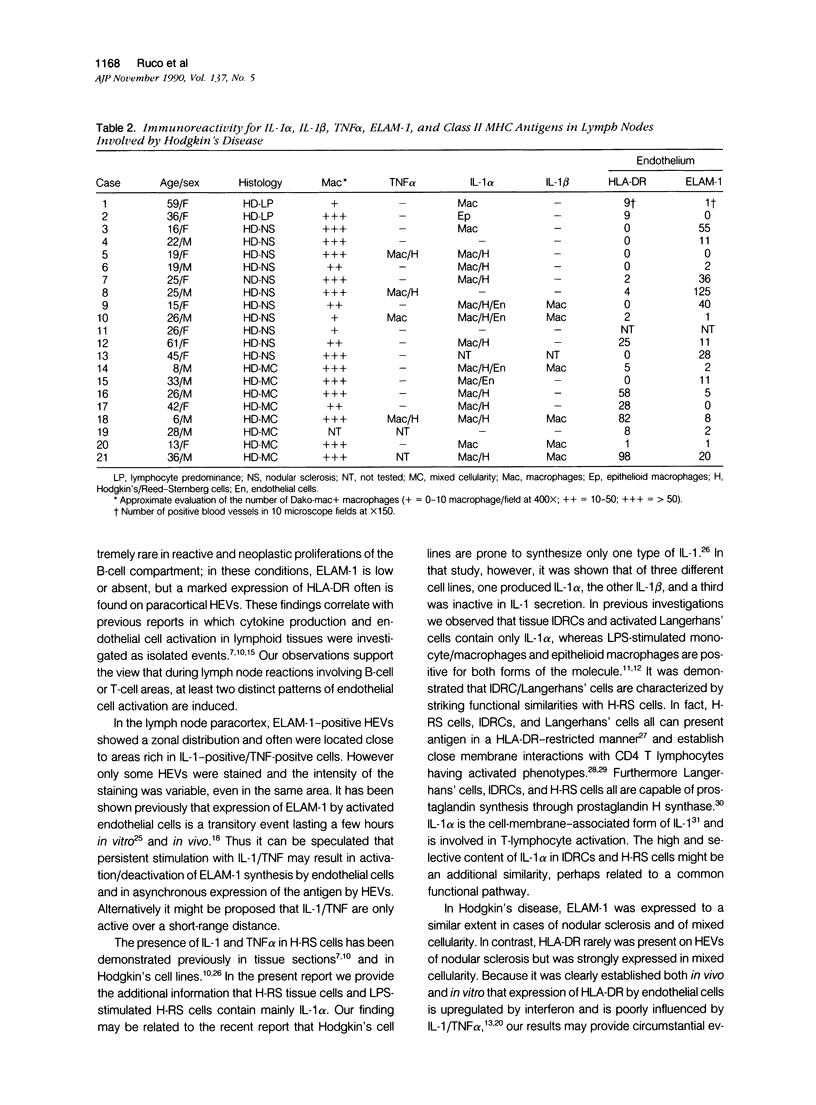
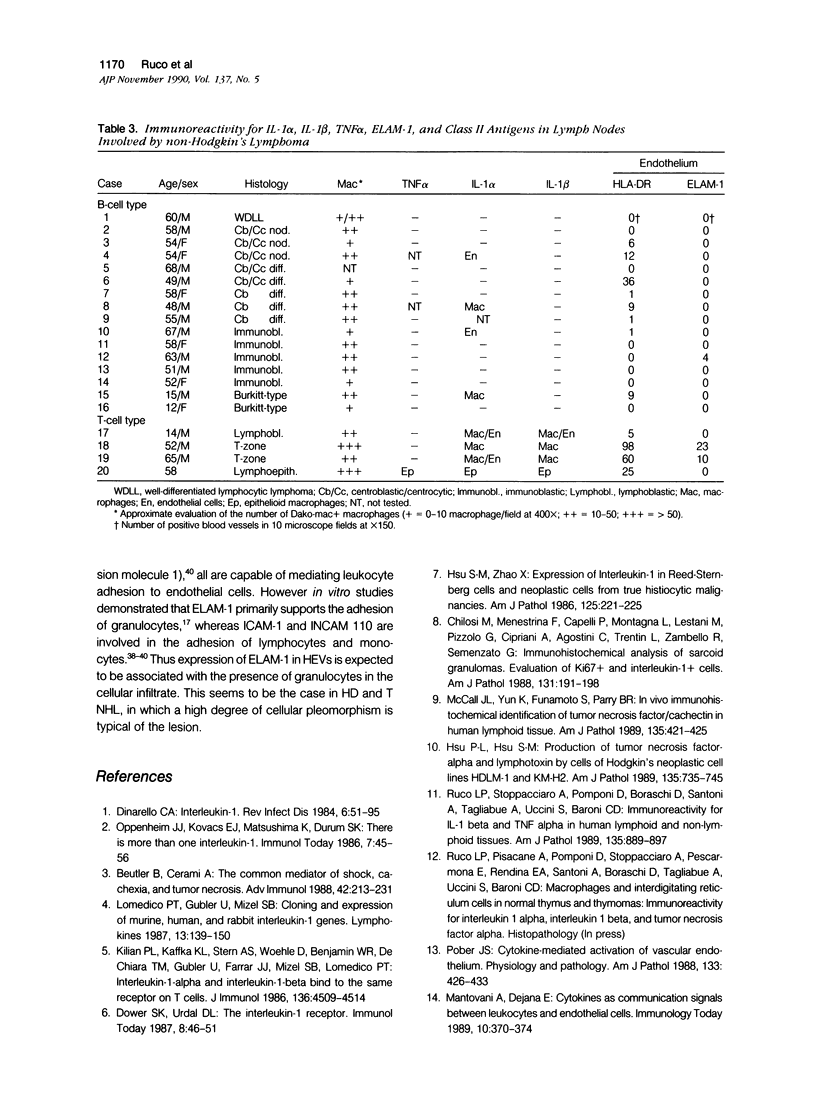
Cryostat sections of 58 lymph nodes were immunostained with a polyclonal rabbit serum against IL-1α, and with monoclonal antibodies directed to IL-1α (Vmp18), IL-1β (Vhp20 and BRhC3), and tumor necrosis factor α (TNFα) (B154.7). Furthermore the presence of cytokine-containing cells was correlated with the expression of endothelial leukocyte adhesion molecule (ELAM-1; 29F2) and of human leukocyte antigen (HLA-DR) (OKIa-1)by endothelial cells. Cells containing IL-1 and/or TNFα were detected mainly inpathologic conditions characterized by reactive or neoplastic expansion of the lymph node paracortex. Cells positive for IL-1 were detected in 16 of 21 cases of Hodgkin's disease,in 4 of 4 cases of T-NHL, and in 5 cases of diffuse or mixed lymphadenitis. Interleukin-1α was detected in macrophages, interdigitating reticulum cells (IDRCs), endothelial cells, and neoplastic Hodgkin's and Reed-Sternberg (H-RS) cells. Cells positive for IL-1 β were much fewer and consisted mainly of macrophages. Hodgkin's Reed-Sternberg cells were negative for IL-1β even after in vitro stimulation with bacterial endotoxin. Tumor necrosis factor α (TNFα) was present in macrophages and H-RS cells. Endothelial leukocyte adhesion molecule-1 expression by endothelial venules was detected in 17 of 20 cases of Hodgkin's disease, in 2 of 4 cases of T-NHL, and in 5 of 5 cases of diffuse lymphadenitis. In these pathologic conditions, HLADR antigens also were expressed frequently by endothelial cells. Cytokine-containing cells and ELAM-1-positive high endothelial venules (HEV) were extremely rare in lymph nodes involved by follicular lymphadenitis (12 cases) or B-NHL (16 cases). In cases of reactive or neoplastic B-cell proliferations, HLA-DR-positive HEVs still were present often. Our results indicate that IL-1/TNFα production at tissue level is often associated with ELAM-1 expression by HEVs, but is less well correlated with expression of HLA-DR antigens by endothelial cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroni C. D., Pezzella F., Stoppacciaro A., Mirolo M., Pescarmona E., Vitolo D., Cassano A. M., Barsotti P., Nicoletti L., Ruco L. P. Systemic lymphadenopathy (LAS) in intravenous drug abusers. Histology, immunohistochemistry and electron microscopy: pathogenic correlations. Histopathology. 1985 Dec;9(12):1275–1293. doi: 10.1111/j.1365-2559.1985.tb02810.x. [DOI] [PubMed] [Google Scholar]
- Bertoglio J. H. B-cell-derived human interleukin 1. Crit Rev Immunol. 1988;8(4):299–313. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The common mediator of shock, cachexia, and tumor necrosis. Adv Immunol. 1988;42:213–231. doi: 10.1016/s0065-2776(08)60846-9. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigio M., Rossi R., Borri M. G., Casagli M. C., Nucci D., Baldari C., Volpini G., Boraschi D. One-step immunoaffinity purification of bioactive human recombinant IL-1 beta with a monoclonal antibody directed to a well-exposed domain of the protein. J Immunol Methods. 1989 Sep 29;123(1):1–8. doi: 10.1016/0022-1759(89)90023-9. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Volpini G., Villa L., Nencioni L., Scapigliati G., Nucci D., Antoni G., Matteucci G., Cioli F., Tagliabue A. A monoclonal antibody to the IL-1 beta peptide 163-171 blocks adjuvanticity but not pyrogenicity of IL-1 beta in vivo. J Immunol. 1989 Jul 1;143(1):131–134. [PubMed] [Google Scholar]
- Chilosi M., Menestrina F., Capelli P., Montagna L., Lestani M., Pizzolo G., Cipriani A., Agostini C., Trentin L., Zambello R. Immunohistochemical analysis of sarcoid granulomas. Evaluation of Ki67+ and interleukin-1+ cells. Am J Pathol. 1988 May;131(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuturi M. C., Murphy M., Costa-Giomi M. P., Weinmann R., Perussia B., Trinchieri G. Independent regulation of tumor necrosis factor and lymphotoxin production by human peripheral blood lymphocytes. J Exp Med. 1987 Jun 1;165(6):1581–1594. doi: 10.1084/jem.165.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Fisher R. I., Cossman J., Diehl V., Volkman D. J. Antigen presentation by Hodgkin's disease cells. J Immunol. 1985 Nov;135(5):3568–3571. [PubMed] [Google Scholar]
- Hirschberg H., Braathen L. R., Thorsby E. Antigen presentation by vascular endothelial cells and epidermal Langerhans cells: the role of HLA-DR. Immunol Rev. 1982;66:57–77. doi: 10.1111/j.1600-065x.1982.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Hsu P. L., Hsu S. M. Production of tumor necrosis factor-alpha and lymphotoxin by cells of Hodgkin's neoplastic cell lines HDLM-1 and KM-H2. Am J Pathol. 1989 Oct;135(4):735–745. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L. Lack of effect of colony-stimulating factors, interleukins, interferons, and tumor necrosis factor on the growth and differentiation of cultured Reed-Sternberg cells. Comparison with effects of phorbol ester and retinoic acid. Am J Pathol. 1990 Jan;136(1):181–189. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L., Lo S. S., Wu K. K. Expression of prostaglandin H synthase (cyclooxygenase) in Hodgkin's mononuclear and Reed-Sternberg cells. Functional resemblance between H-RS cells and histiocytes or interdigitating reticulum cells. Am J Pathol. 1988 Oct;133(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Krupen K., Lachman L. B. Heterogeneity of interleukin 1 production in cultured Reed-Sternberg cell lines HDLM-1, HDLM-1d, and KM-H2. Am J Pathol. 1989 Jul;135(1):33–38. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. Expression of interleukin-1 in Reed-Sternberg cells and neoplastic cells from true histiocytic malignancies. Am J Pathol. 1986 Nov;125(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Kilian P. L., Kaffka K. L., Stern A. S., Woehle D., Benjamin W. R., Dechiara T. M., Gubler U., Farrar J. J., Mizel S. B., Lomedico P. T. Interleukin 1 alpha and interleukin 1 beta bind to the same receptor on T cells. J Immunol. 1986 Jun 15;136(12):4509–4514. [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- McCall J. L., Yun K., Funamoto S., Parry B. R. In vivo immunohistochemical identification of tumor necrosis factor/cachectin in human lymphoid tissue. Am J Pathol. 1989 Sep;135(3):421–425. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Tumor necrosis factor and interferon-gamma induce distinct patterns of endothelial activation and associated leukocyte accumulation in skin of Papio anubis. Am J Pathol. 1989 Jul;135(1):121–133. [PMC free article] [PubMed] [Google Scholar]
- Naumovski L., Utz P. J., Bergstrom S. K., Morgan R., Molina A., Toole J. J., Glader B. E., McFall P., Weiss L. M., Warnke R. SUP-HD1: a new Hodgkin's disease-derived cell line with lymphoid features produces interferon-gamma. Blood. 1989 Dec;74(8):2733–2742. [PubMed] [Google Scholar]
- Numerof R. P., Aronson F. R., Mier J. W. IL-2 stimulates the production of IL-1 alpha and IL-1 beta by human peripheral blood mononuclear cells. J Immunol. 1988 Dec 15;141(12):4250–4257. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S. Warner-Lambert/Parke-Davis award lecture. Cytokine-mediated activation of vascular endothelium. Physiology and pathology. Am J Pathol. 1988 Dec;133(3):426–433. [PMC free article] [PubMed] [Google Scholar]
- Poppema S., Bhan A. K., Reinherz E. L., Posner M. R., Schlossman S. F. In situ immunologic characterization of cellular constituents in lymph nodes and spleens involved by Hodgkin's disease. Blood. 1982 Feb;59(2):226–232. [PubMed] [Google Scholar]
- Poppema S. The nature of the lymphocytes surrounding Reed-Sternberg cells in nodular lymphocyte predominance and in other types of Hodgkin's disease. Am J Pathol. 1989 Aug;135(2):351–357. [PMC free article] [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruco L. P., Stoppacciaro A., Pomponi D., Boraschi D., Santoni A., Tagliabue A., Uccini S., Baroni C. D. Immunoreactivity for IL-1 beta and TNF alpha in human lymphoid and nonlymphoid tissues. Am J Pathol. 1989 Nov;135(5):889–897. [PMC free article] [PubMed] [Google Scholar]
- Samoszuk M., Nansen L. Detection of interleukin-5 messenger RNA in Reed-Sternberg cells of Hodgkin's disease with eosinophilia. Blood. 1990 Jan 1;75(1):13–16. [PubMed] [Google Scholar]
- Sappino A. P., Seelentag W., Pelte M. F., Alberto P., Vassalli P. Tumor necrosis factor/cachectin and lymphotoxin gene expression in lymph nodes from lymphoma patients. Blood. 1990 Feb 15;75(4):958–962. [PubMed] [Google Scholar]