Abstract
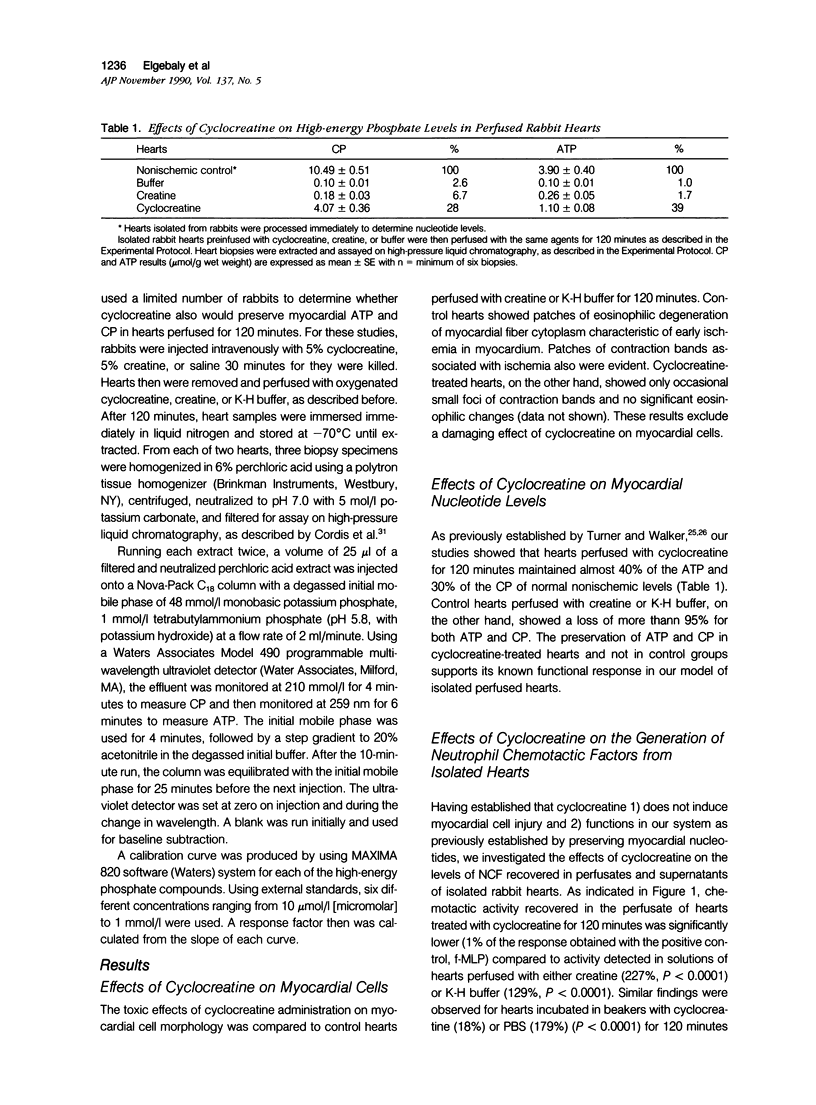
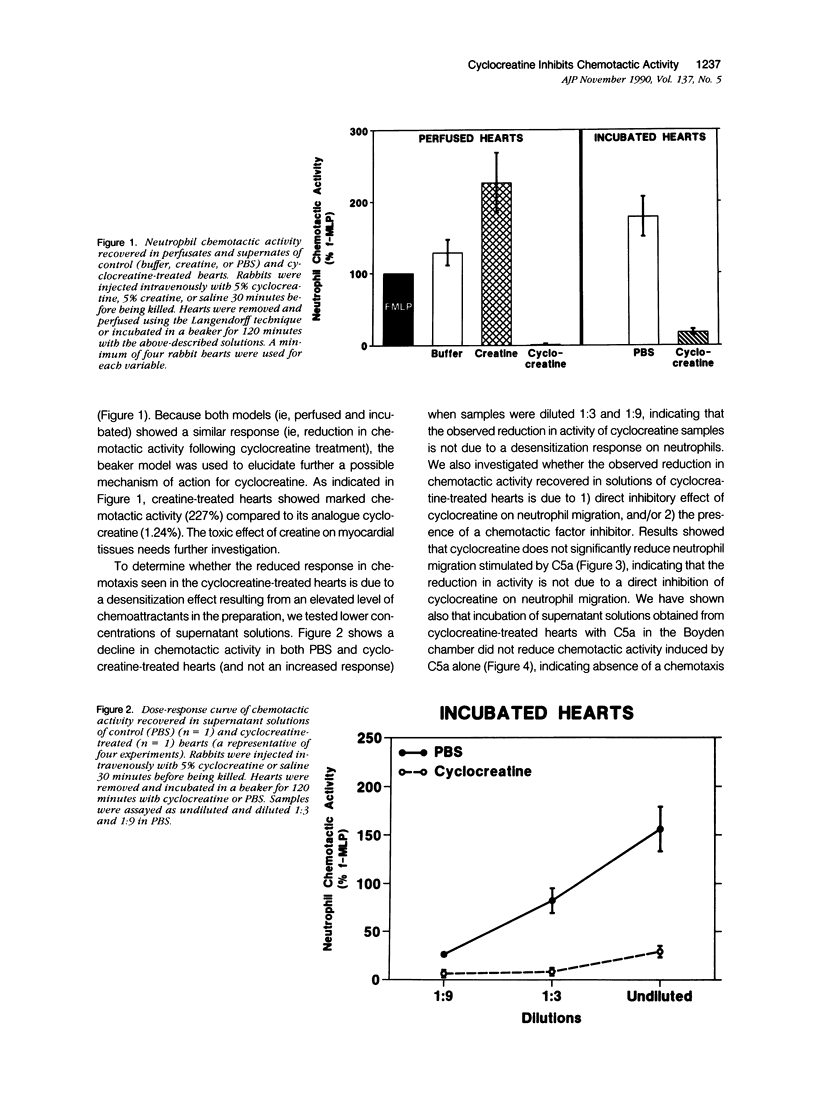
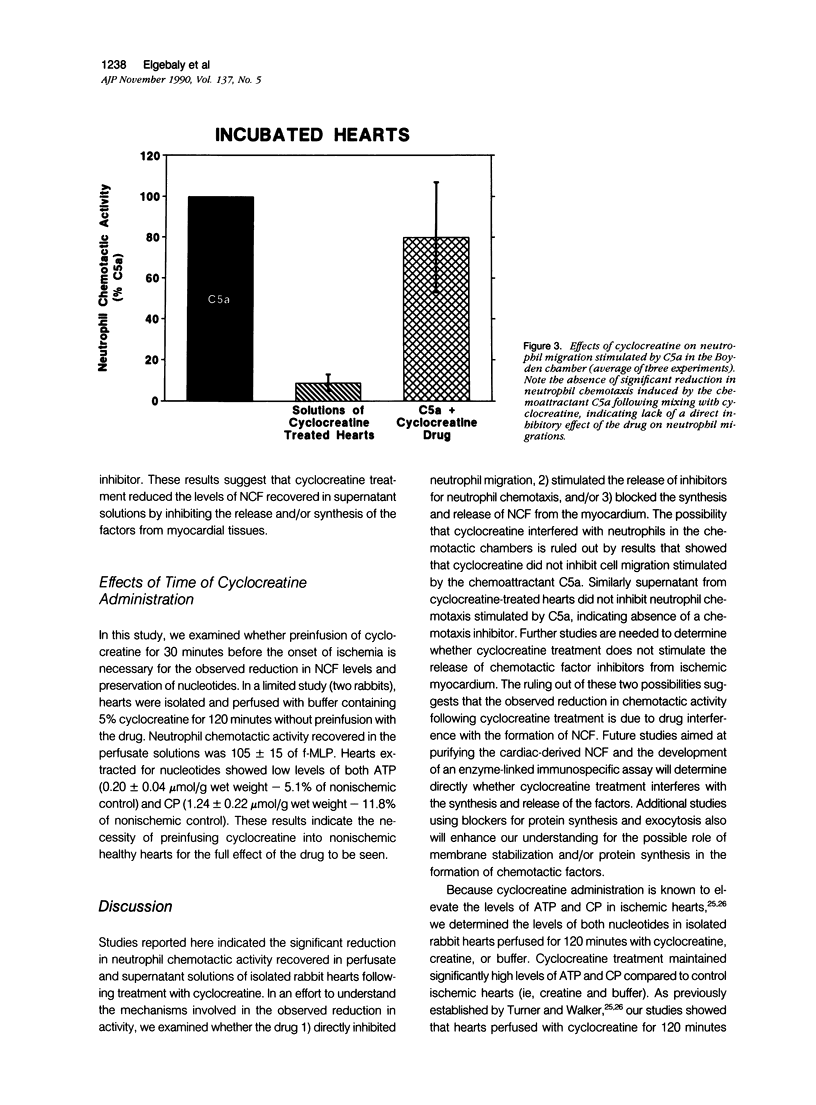
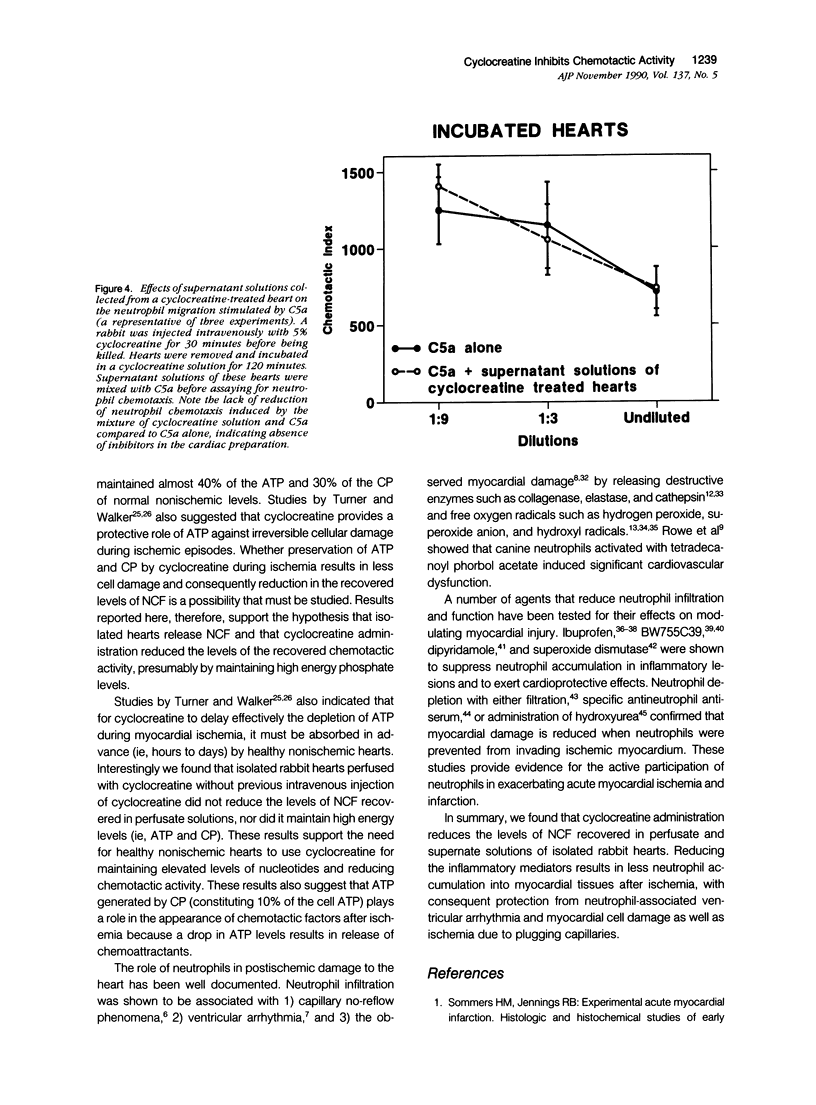
This study was designed to determine the effect of cyclocreatine on the release of neutrophil chemotactic factors (NCF) from isolated rabbit hearts. We tested the hypothesis that if ischemia is important for the formation of NCF from the myocardium, then blocking (or delaying) ischemic changes with cyclocreatine should inhibit the release of NCF. Two models were used, including (1) perfusion of rabbit hearts (Langendorff apparatus) with oxygenated (95% oxygen) Krebs-Henseleit buffer (K-H buffer) containing 5% cyclocreatine for 120 minutes, and (2) incubating hearts with phosphate-buffered saline (PBS) containing 5% cyclocreatine for 120 minutes. For both models, rabbits were injected intravenously with 10 ml of 5% cyclocreatine solution 30 minutes before the animals were killed and the hearts removed. Control rabbits were injected with 5% creatine solution or saline for 30 minutes before perfusing hearts with K-H buffer or incubating with PBS. Chemotactic activity was assayed in the perfusates and supernatants using modified Boyden chambers and rabbit peritoneal neutrophils as indicator cells. The chemoattractant f-Met-Leu-Phe (f-MLP) was the positive control for a 100% response rate. Isolated hearts perfused with cyclocreatine showed significantly lower chemotactic activity (ie, 1.24 +/- 1% f-MLP; P less than 0.0001) compared to hearts perfused with K-H buffer (129 +/- 18%) or creatine (227 +/- 42%) (mean +/- standard error). Similar results were obtained using incubated hearts. Next the effect of cyclocreatine on neutrophils in the Boyden chamber was determined and it was found that it did not alter neutrophil migration, which excludes a direct inhibitory effect on the cells. Furthermore supernatant from cyclocreatine-treated hearts did not inhibit neutrophil chemotaxis to C5a, indicating absence of a chemotaxis inhibitor in this preparation. Results of these studies suggest that the observed low activity recovered in perfusate and supernatant of cyclocreatine-treated hearts is a result of reduction in the synthesis and/or release of the factors from myocardial tissues. Similar to previously established data, cyclocreatine treatment significantly preserved myocardial nucleotide levels (ie, adenosine triphosphate and creatine phosphate), which supports our hypothesis that the formation of NCF is ischemia dependent and that maintaining elevated levels of myocardial energy nucleotides reduced chemotactic factor release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annesley T. M., Walker J. B. Energy metabolism of skeletal muscle containing cyclocreatine phosphate. Delay in onset of rigor mortis and decreased glycogenolysis in response to ischemia or epinephrine. J Biol Chem. 1980 May 10;255(9):3924–3930. [PubMed] [Google Scholar]
- Bell D., Jackson M., Millar A. M., Nicoll J. J., Connell M., Muir A. L. The acute inflammatory response to myocardial infarction: imaging with indium-111 labelled autologous neutrophils. Br Heart J. 1987 Jan;57(1):23–27. doi: 10.1136/hrt.57.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell P. R., Camussi G., Brentjens J. R., Andres G. Lung injury in rabbits induced by intravenous administration of heterologous polyclonal antibodies to angiotensin converting enzyme (kininase II). J Mol Cell Cardiol. 1989 Feb;21 (Suppl 1):171–174. doi: 10.1016/0022-2828(89)90854-7. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Yuen C., Perez H. D., Goldstein I. M. Chemotactic peptides modulate adherence of human polymorphonuclear leukocytes to monolayers of cultured endothelial cells. J Immunol. 1986 May 1;136(9):3412–3419. [PubMed] [Google Scholar]
- Cordis G. A., Engelman R. M., Das D. K. Novel dual-wavelength monitoring approach for the improved rapid separation and estimation of adenine nucleotides and creatine phosphate by high-performance liquid chromatography. J Chromatogr. 1988 Dec 28;459:229–236. doi: 10.1016/s0021-9673(01)82031-8. [DOI] [PubMed] [Google Scholar]
- Davies R. A., Thakur M. L., Berger H. J., Wackers F. J., Gottschalk A., Zaret B. L. Imaging the inflammatory response to acute myocardial infarction in man using indium-111-labeled autologous platelets. Circulation. 1981 Apr;63(4):826–832. doi: 10.1161/01.cir.63.4.826. [DOI] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgebaly S. A., Downes R. T., Bohr M., Forouhar F., O'Rourke J., Kreutzer D. L. Inflammatory mediators in alkali-burned corneas: preliminary characterization. Curr Eye Res. 1987 Nov;6(11):1263–1274. doi: 10.3109/02713688708997551. [DOI] [PubMed] [Google Scholar]
- Elgebaly S. A., Herkert N., O'Rourke J., Kreutzer D. L. Characterization of neutrophil and monocyte specific chemotactic factors derived from the cornea in response to hydrogen peroxide injury. Am J Pathol. 1987 Jan;126(1):40–50. [PMC free article] [PubMed] [Google Scholar]
- Elgebaly S. A., Masetti P., Allam M., Forouhar F. Cardiac derived neutrophil chemotactic factors; preliminary biochemical characterization. J Mol Cell Cardiol. 1989 Jun;21(6):585–593. doi: 10.1016/0022-2828(89)90824-9. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Engler R. Consequences of activation and adenosine-mediated inhibition of granulocytes during myocardial ischemia. Fed Proc. 1987 May 15;46(7):2407–2412. [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. R., Walker J. B. Accumulation of analgo of phosphocreatine in muscle of chicks fed 1-carboxymethyl-2-iminoimidazolidine (cyclocreatine). J Biol Chem. 1976 Apr 10;251(7):2049–2054. [PubMed] [Google Scholar]
- Hess M. L., Rowe G. T., Caplan M., Romson J. L., Lucchesi B. Identification of hydrogen peroxide and hydroxyl radicals as mediators of leukocyte-induced myocardial dysfunction. Limitation of infarct size with neutrophil inhibition and depletion. Adv Myocardiol. 1985;5:159–175. [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Jolly S. R., Lucchesi B. R. Effect of BW755C in an occlusion-reperfusion model of ischemic myocardial injury. Am Heart J. 1983 Jul;106(1 Pt 1):8–13. doi: 10.1016/0002-8703(83)90431-3. [DOI] [PubMed] [Google Scholar]
- Jugdutt B. I., Hutchins G. M., Bulkley B. H., Becker L. C. Salvage of ischemic myocardium by ibuprofen during infarction in the conscious dog. Am J Cardiol. 1980 Jul;46(1):74–82. doi: 10.1016/0002-9149(80)90608-6. [DOI] [PubMed] [Google Scholar]
- Kozol R. A., Downes R. J., Kreutzer D. L., Wentzel S., Rossomando E., Elgebaly S. A. Release of neutrophil chemotactic factors from gastric tissue. Initial biochemical characterization. Dig Dis Sci. 1989 May;34(5):681–687. doi: 10.1007/BF01540338. [DOI] [PubMed] [Google Scholar]
- Kozol R. A., Punzo A., Ribaudo R., Rossomando E. F., Elgebaly S. A. Neutrophil chemotactic activity in human gastric secretions. Arch Surg. 1990 Apr;125(4):454–456. doi: 10.1001/archsurg.1990.01410160040008. [DOI] [PubMed] [Google Scholar]
- Lucchesi B. R., Mullane K. M. Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Moncada S. The salvage of ischaemic myocardium by BW755C in anaesthetised dogs. Prostaglandins. 1982 Aug;24(2):255–266. doi: 10.1016/0090-6980(82)90151-4. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Neely J. R., Rovetto M. J. Techniques for perfusing isolated rat hearts. Methods Enzymol. 1975;39:43–60. doi: 10.1016/s0076-6879(75)39008-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. J., Walker J. B. Feeding a creatine analogue delays ATP depletion and onset of rigor in ischemic heart. Am J Physiol. 1982 Dec;243(6):H911–H916. doi: 10.1152/ajpheart.1982.243.6.H911. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Bush L. R., Haack D. W., Lucchesi B. R. The beneficial effects of oral ibuprofen on coronary artery thrombosis and myocardial ischemia in the conscious dog. J Pharmacol Exp Ther. 1980 Oct;215(1):271–278. [PubMed] [Google Scholar]
- Romson J. L., Bush L. R., Jolly S. R., Lucchesi B. R. Cardioprotective effects of ibuprofen in experimental regional and global myocardial ischemia. J Cardiovasc Pharmacol. 1982 Mar-Apr;4(2):187–196. doi: 10.1097/00005344-198203000-00005. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rowe G. T., Eaton L. R., Hess M. L. Neutrophil-derived, oxygen free radical-mediated cardiovascular dysfunction. J Mol Cell Cardiol. 1984 Nov;16(11):1075–1079. doi: 10.1016/s0022-2828(84)80020-6. [DOI] [PubMed] [Google Scholar]
- Ruby S. T., Allam M. E., Gallo M. A., Barth S., Elgebaly S. A. Release of chemotactic factors by veins during preparation for arterial bypass. Arch Surg. 1990 Apr;125(4):481–484. doi: 10.1001/archsurg.1990.01410160067014. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc. 1987 May 15;46(7):2397–2401. [PubMed] [Google Scholar]
- Teoh K. H., Christakis G. T., Weisel R. D., Madonik M. M., Ivanov J., Warbick-Cerone A., Johnston L. G., Cawthorn R. H., Mullen J. C., Glynn M. F. Dipyridamole reduced myocardial platelet and leukocyte deposition following ischemia and cardioplegia. J Surg Res. 1987 Jun;42(6):642–652. doi: 10.1016/0022-4804(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Ts'ao C., Lin C. Y., Glagov S., Replogle R. L. Disseminated leukocyte injury during open-heart surgery. Arch Pathol. 1973 Jun;95(6):357–365. [PubMed] [Google Scholar]
- Turner D. M., Walker J. B. Enhanced ability of skeletal muscle containing cyclocreatine phosphate to sustain ATP levels during ischemia following beta-adrenergic stimulation. J Biol Chem. 1987 May 15;262(14):6605–6609. [PubMed] [Google Scholar]
- Turner D. M., Walker J. B. Relative abilities of phosphagens with different thermodynamic or kinetic properties to help sustain ATP and total adenylate pools in heart during ischemia. Arch Biochem Biophys. 1985 May 1;238(2):642–651. doi: 10.1016/0003-9861(85)90210-3. [DOI] [PubMed] [Google Scholar]
- Woznicki D. T., Walker J. B. Utilization of cyclocreatine phosphate, and analogue of creatine phosphate, by mouse brain during ischemia and its sparing action on brain energy reserves. J Neurochem. 1980 May;34(5):1247–1253. doi: 10.1111/j.1471-4159.1980.tb09966.x. [DOI] [PubMed] [Google Scholar]


