Abstract
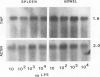
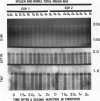
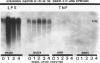
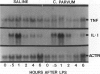
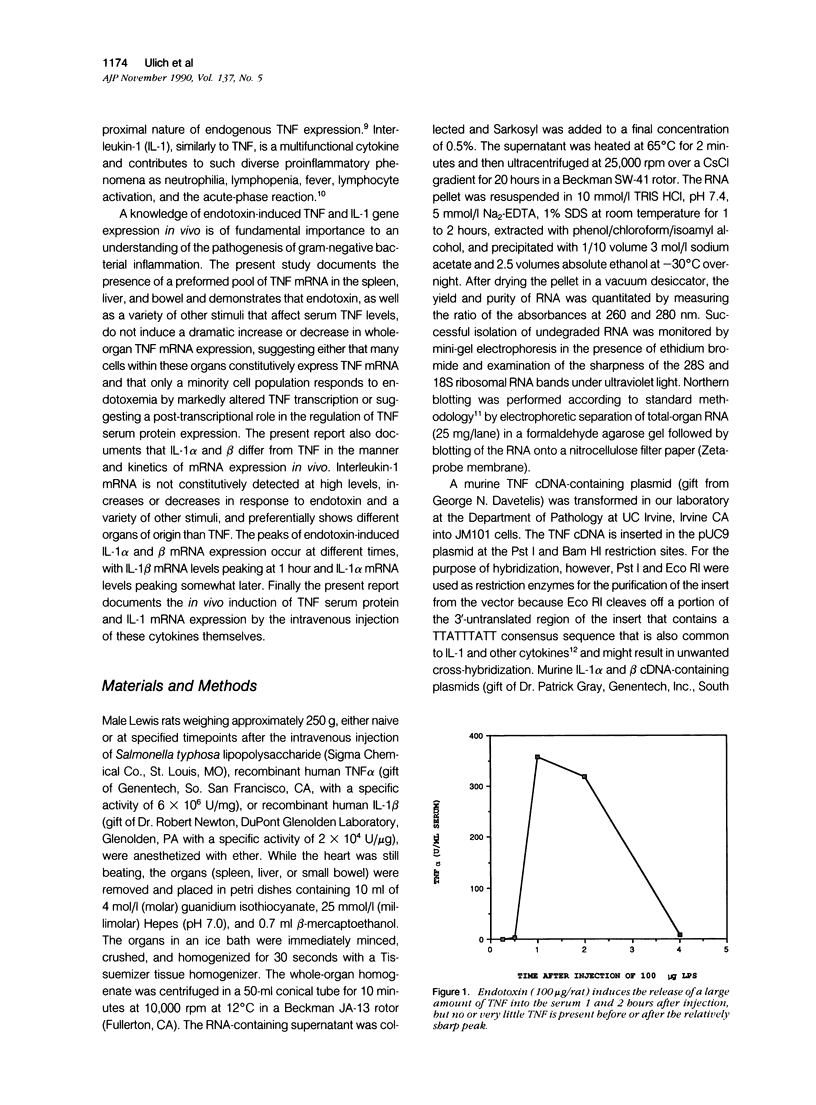
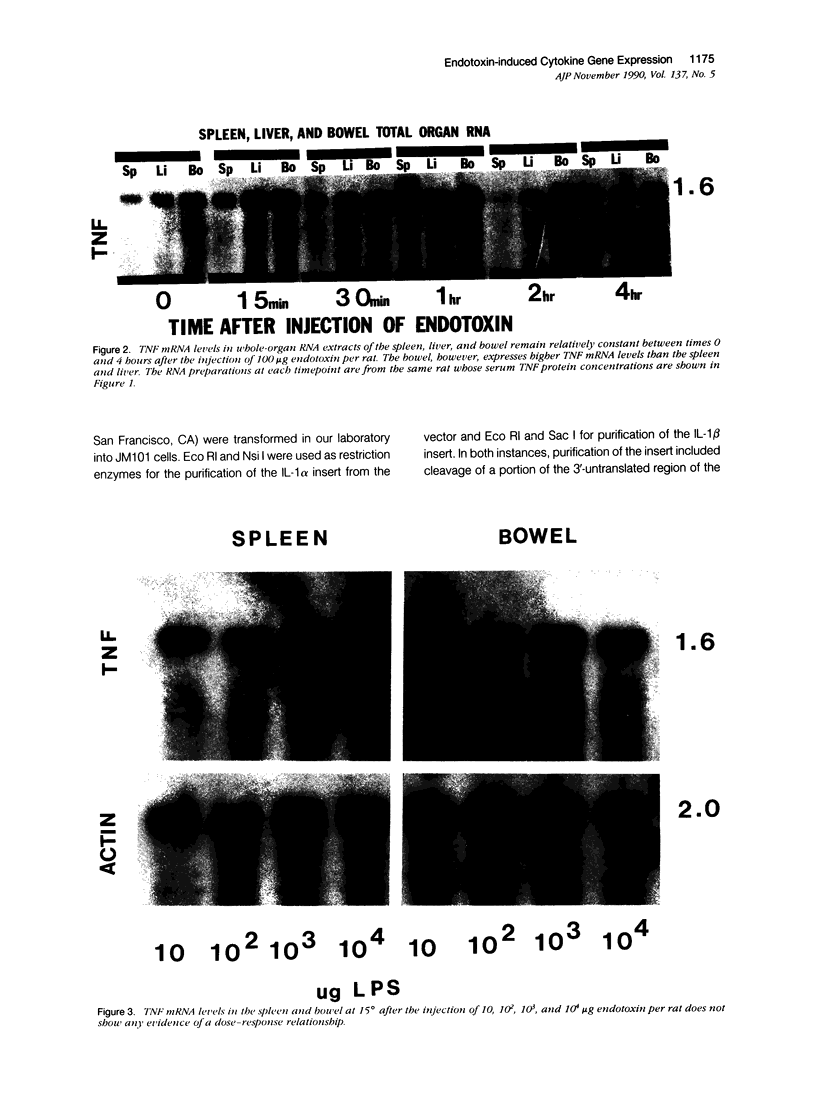
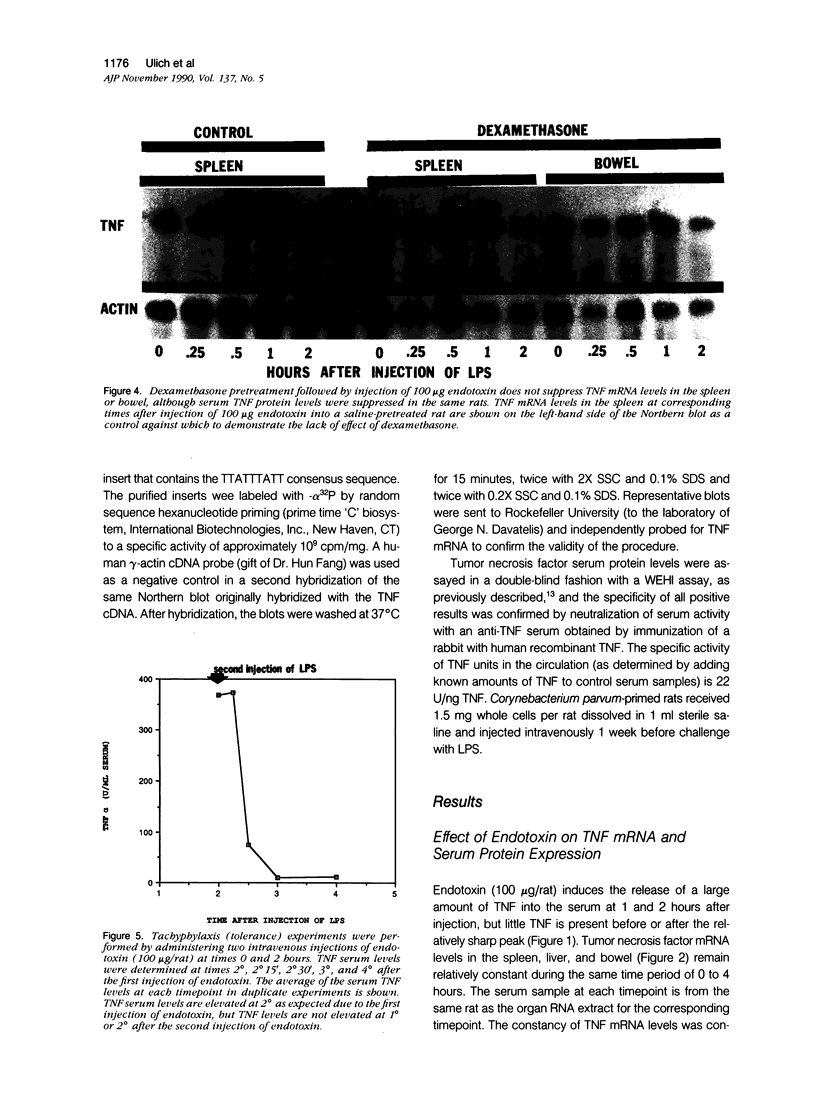
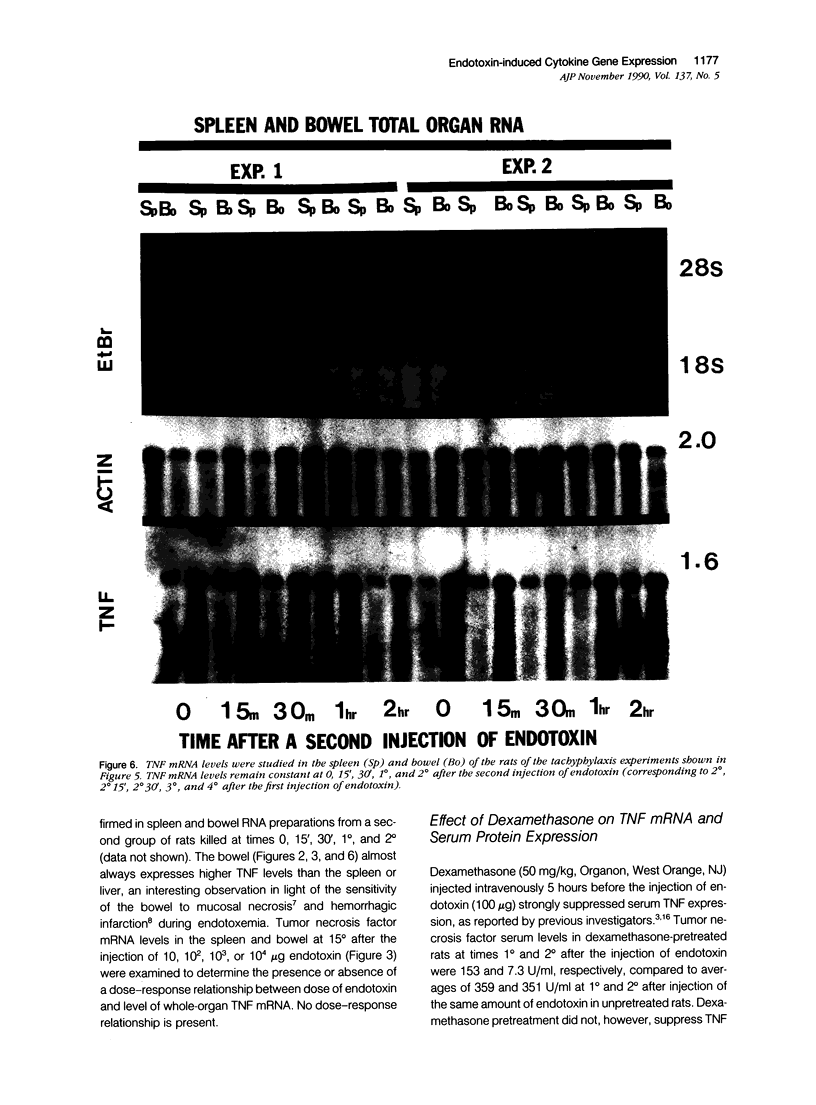
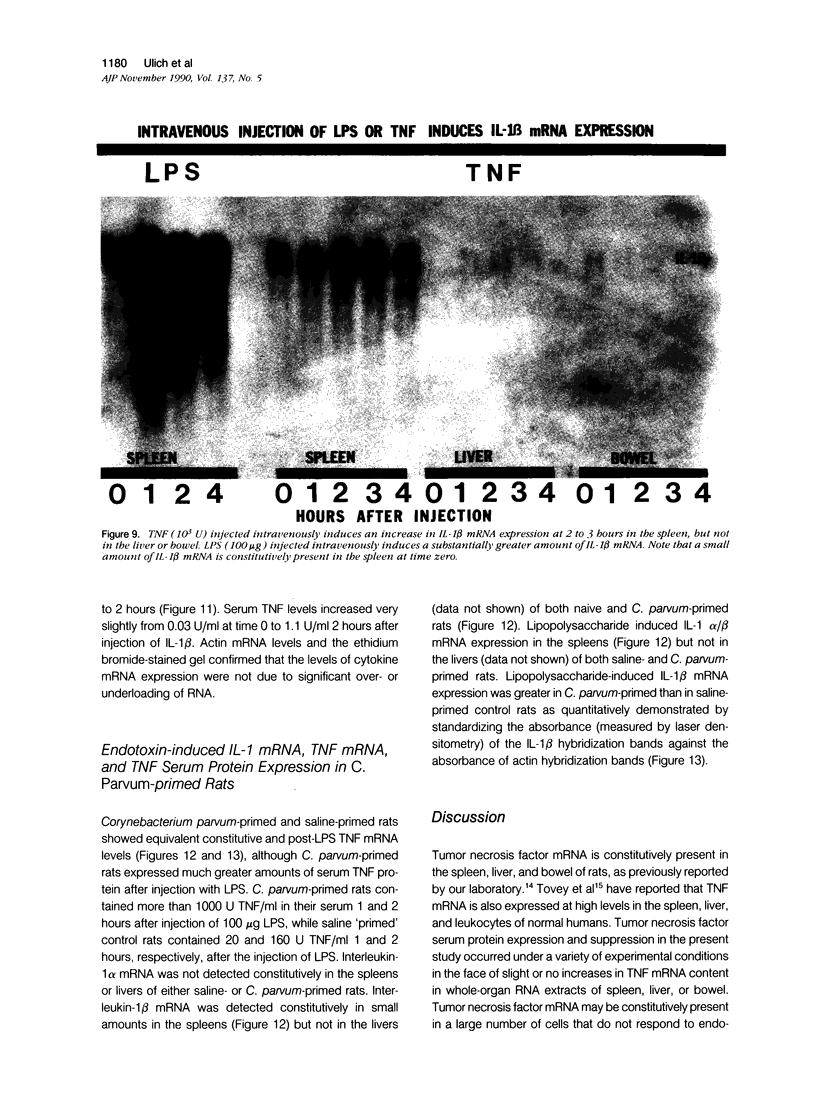
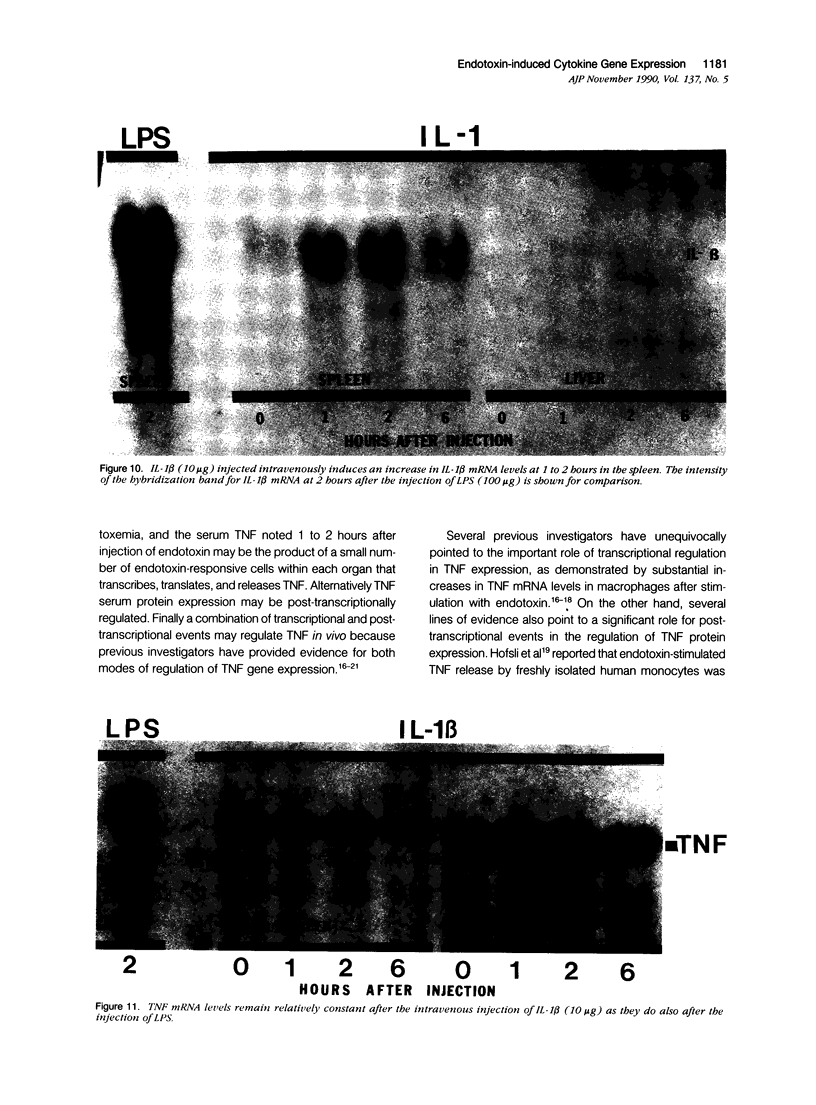
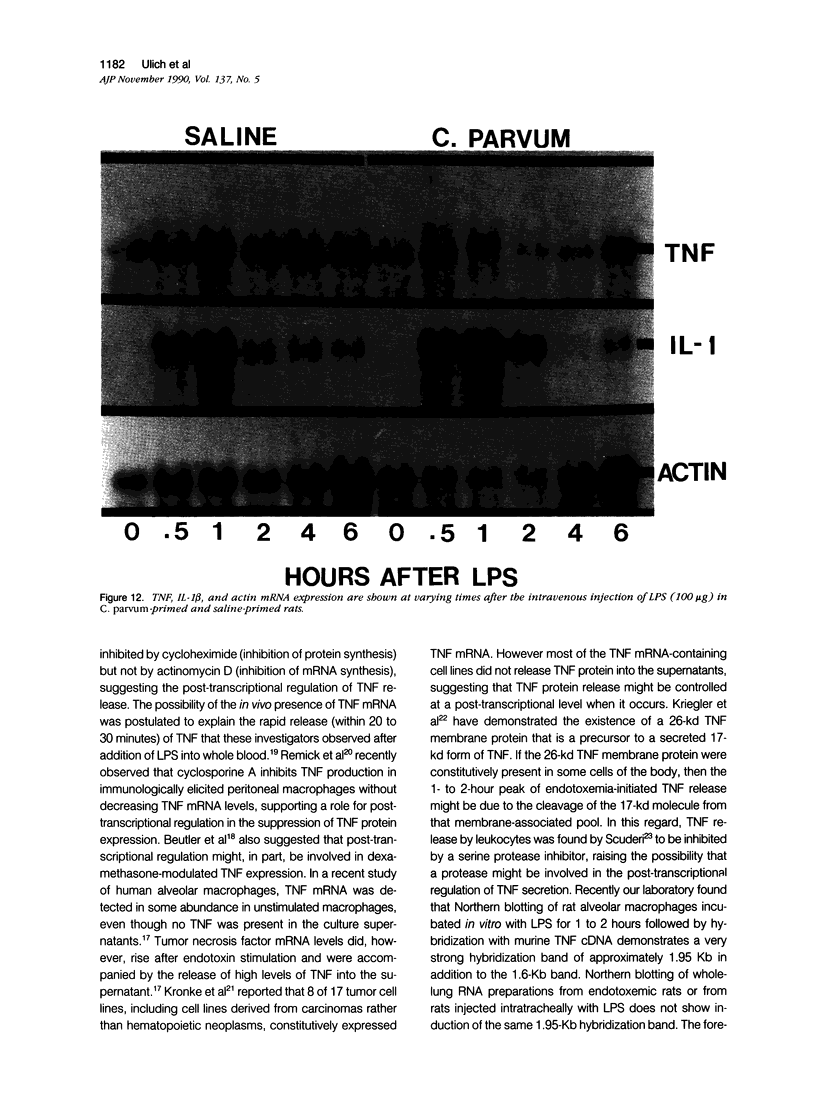
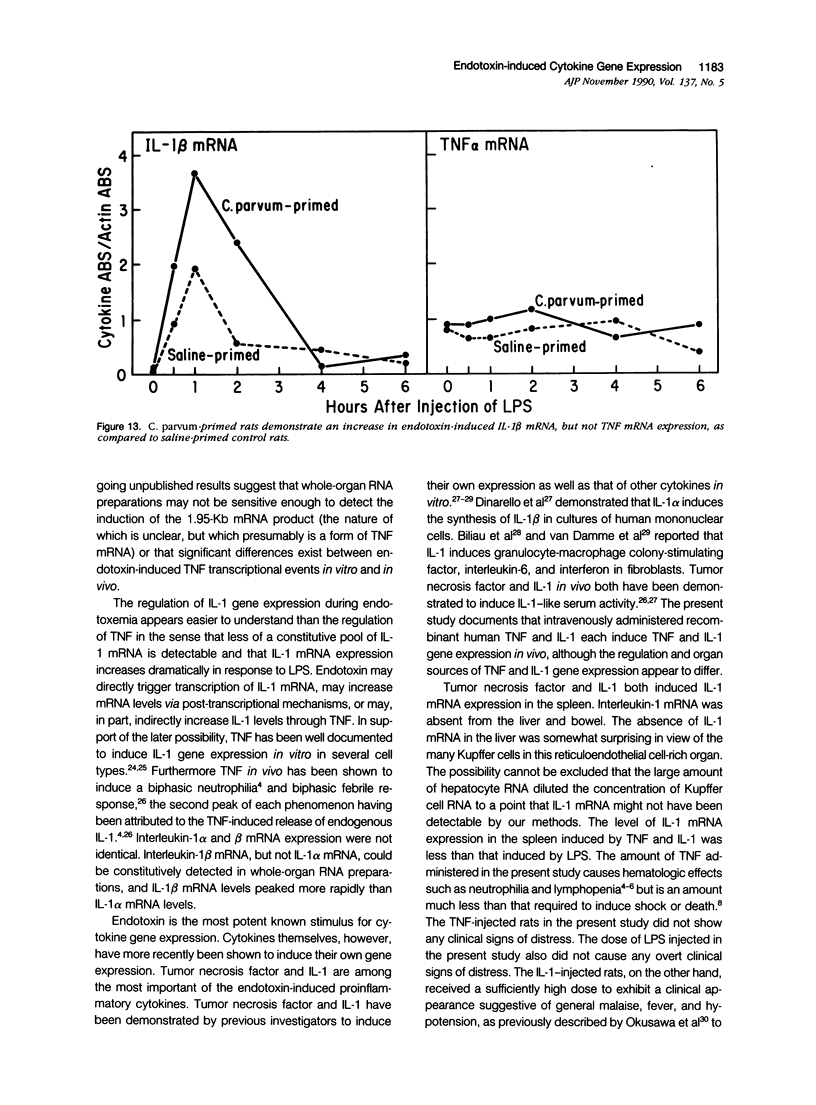
Tumor necrosis factor alpha (TNF alpha) mRNA is present in a preformed intracellular pool in the spleen, liver, and small bowel of naive rats. Endotoxin (Salmonella typhus lipopolysaccharide) injected intravenously induces little or no increase in whole-organ TNF mRNA levels at 15', 30', 1 degree, 2 degrees, or 4 degrees, whereas serum TNF levels are markedly elevated at 1 and 2 hours. Dexamethasone pretreatment of rats suppresses LPS-induced serum TNF concentrations, but does not suppress TNF mRNA levels in the spleen or bowel. Tachyphylaxis experiments demonstrate that a second injection of endotoxin 2 hours after an initial injection fails to induce a second peak of serum TNF, although TNF mRNA levels in the spleen and bowel remain at the levels found in naive rats. Corynebacterium parvum upregulates endotoxin-induced serum TNF release and intravenous injection of IL-1 induces the release of serum TNF but neither alters whole-organ TNF mRNA levels. Interleukin-1 alpha (IL-1 alpha) mRNA was not constitutively detected in whole-organ RNA preparations of the spleen, liver, and small bowel of naive rats. Endotoxin induces IL-1 alpha mRNA most easily appreciated in the spleen beginning at 1 hour, peaking at 2 to 4 hours, and disappearing by 6 hours. Interleukin-1 beta (IL-1 beta) mRNA was not constitutively detected in the organs examined or was present in small amounts. Endotoxin induces IL-1 beta mRNA beginning at 0.5 hours, peaking at 1 hour, and disappearing by 6 hours. Dexamethasone pretreatment prevents the LPS-induced appearance of IL-1 alpha mRNA and suppresses but does not completely inhibit the appearance of IL-1 beta mRNA. C. parvum upregulates endotoxin-induced IL-1 mRNA expression. Intravenous injection of TNF or IL-1 both induce IL-1 mRNA expression. In conclusion, TNF mRNA is constitutively expressed and TNF mRNA levels as analyzed in whole-organ RNA preparations do not change in concert with serum TNF protein levels during conditions of endotoxemia, dexamethasone treatment, tachyphylaxis, priming with C. parvum, or after injection of IL-1. In contrast, IL-1 mRNA expression during endotoxemia, dexamethasone treatment, priming with C. parvum, or after injection of TNF or IL-1 shows clear increases and decreases in whole-organ RNA preparations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Becker S., Devlin R. B., Haskill J. S. Differential production of tumor necrosis factor, macrophage colony stimulating factor, and interleukin 1 by human alveolar macrophages. J Leukoc Biol. 1989 Apr;45(4):353–361. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Billiau A., Van Damme J., Opdenakker G., Fibbe W. E., Falkenburg J. H., Content J. Interleukin 1 as a cytokine inducer. Immunobiology. 1986 Sep;172(3-5):323–335. doi: 10.1016/S0171-2985(86)80114-0. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Chan M. K., Movat H. Z. Acute inflammation and microthrombosis induced by endotoxin, interleukin-1, and tumor necrosis factor and their implication in gram-negative infection. Lab Invest. 1988 Apr;58(4):365–378. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Ikejima T., Warner S. J., Orencole S. F., Lonnemann G., Cannon J. G., Libby P. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987 Sep 15;139(6):1902–1910. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Hofsli E., Lamvik J., Nissen-Meyer J. Evidence that tumour necrosis factor (TNF) is not constitutively present in vivo. The association of TNF with freshly isolated monocytes reflects a rapid in vitro production. Scand J Immunol. 1988 Oct;28(4):435–441. doi: 10.1111/j.1365-3083.1988.tb01473.x. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kruys V., Marinx O., Shaw G., Deschamps J., Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989 Aug 25;245(4920):852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- Krönke M., Hensel G., Schlüter C., Scheurich P., Schütze S., Pfizenmaier K. Tumor necrosis factor and lymphotoxin gene expression in human tumor cell lines. Cancer Res. 1988 Oct 1;48(19):5417–5421. [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remick D. G., Kunkel R. G., Larrick J. W., Kunkel S. L. Acute in vivo effects of human recombinant tumor necrosis factor. Lab Invest. 1987 Jun;56(6):583–590. [PubMed] [Google Scholar]
- Remick D. G., Nguyen D. T., Eskandari M. K., Strieter R. M., Kunkel S. L. Cyclosporine A inhibits TNF production without decreasing TNF mRNA levels. Biochem Biophys Res Commun. 1989 Jun 15;161(2):551–555. doi: 10.1016/0006-291x(89)92634-x. [DOI] [PubMed] [Google Scholar]
- Remick D. G., Strieter R. M., Lynch J. P., 3rd, Nguyen D., Eskandari M., Kunkel S. L. In vivo dynamics of murine tumor necrosis factor-alpha gene expression. Kinetics of dexamethasone-induced suppression. Lab Invest. 1989 Jun;60(6):766–771. [PubMed] [Google Scholar]
- Scuderi P. Suppression of human leukocyte tumor necrosis factor secretion by the serine protease inhibitor p-toluenesulfonyl-L-arginine methyl ester (TAME). J Immunol. 1989 Jul 1;143(1):168–173. [PubMed] [Google Scholar]
- Tovey M. G., Content J., Gresser I., Gugenheim J., Blanchard B., Guymarho J., Poupart P., Gigou M., Shaw A., Fiers W. Genes for IFN-beta-2 (IL-6), tumor necrosis factor, and IL-1 are expressed at high levels in the organs of normal individuals. J Immunol. 1988 Nov 1;141(9):3106–3110. [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Guo K., del Castillo J. Endotoxin-induced cytokine gene expression in vivo. I. Expression of tumor necrosis factor mRNA in visceral organs under physiologic conditions and during endotoxemia. Am J Pathol. 1989 Jan;134(1):11–14. [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Keys M., Granger G. A., Ni R. X. Kinetics and mechanisms of recombinant human interleukin 1 and tumor necrosis factor-alpha-induced changes in circulating numbers of neutrophils and lymphocytes. J Immunol. 1987 Nov 15;139(10):3406–3415. [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Ni R. X., Bikhazi N., Calvin L. Mechanisms of tumor necrosis factor alpha-induced lymphopenia, neutropenia, and biphasic neutrophilia: a study of lymphocyte recirculation and hematologic interactions of TNF alpha with endogenous mediators of leukocyte trafficking. J Leukoc Biol. 1989 Feb;45(2):155–167. doi: 10.1002/jlb.45.2.155. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Ni R. X., Bikhazi N. Hematologic interactions of endotoxin, tumor necrosis factor alpha (TNF alpha), interleukin 1, and adrenal hormones and the hematologic effects of TNF alpha in Corynebacterium parvum-primed rats. J Leukoc Biol. 1989 Jun;45(6):546–557. doi: 10.1002/jlb.45.6.546. [DOI] [PubMed] [Google Scholar]
- Waage A. Production and clearance of tumor necrosis factor in rats exposed to endotoxin and dexamethasone. Clin Immunol Immunopathol. 1987 Dec;45(3):348–355. doi: 10.1016/0090-1229(87)90087-0. [DOI] [PubMed] [Google Scholar]
- van Damme J., Opdenakker G., de Ley M., Heremans H., Billiau A. Pyrogenic and haematological effects of the interferon-inducing 22K factor (interleukin 1 beta) from human leukocytes. Clin Exp Immunol. 1986 Nov;66(2):303–311. [PMC free article] [PubMed] [Google Scholar]