Abstract
Few treatments for obesity exist and, whereas efficacious therapeutics for hyperlipidemia are available, further improvements are desirable. Thyroid hormone receptors (TRs) regulate both body weight and cholesterol levels. However, thyroid hormones also have deleterious effects, particularly on the heart. The TRβ subtype is involved in cholesterol lowering and possibly elevating metabolic rate, whereas TRα appears to be more important for control of heart rate (HR). In the current studies, we examined the effect of TRβ activation on metabolic rate and HR with either TRα1–/– mice or the selective TRβ agonist KB-141 in mice, rats, and monkeys. 3,5,3′-triiodi-l-thyronine (T3) had a greater effect on increasing HR in WT than in TRα–/– mice (ED15 values of 34 and 469 nmol/kg/day, respectively). T3 increased metabolic rate [whole body oxygen consumption (MVO2)] in both WT and TRα–/– mice, but the effect in the TRα1–/– mice at the highest dose was half that of the WT mice. Thus, stimulation of MVO2 is likely due to both TRα and -β. T3 had equivalent potency for cholesterol reduction in WT and TRα–/– mice. KB-141 increased MVO2 with selectivities of 16.5- and 11.2-fold vs. HR in WT and TRα1–/– mice, respectively. KB-141 also increased MVO2 with a 10-fold selectivity and lowered cholesterol with a 27-fold selectivity vs. HR in rats. In primates, KB-141 caused significant cholesterol, lipoprotein (a), and body-weight reduction (up to 7% after 1 wk) with no effect on HR. TRβ-selective agonists may constitute a previously uncharacterized class of drugs to treat obesity, hypercholesterolemia, and elevated lipoprotein (a).
Obesity and atherosclerosis are important medical problems with major impact on morbidity and mortality. Current treatments for obesity have shown limited efficacy and safety; therefore, there is a need for improved therapies (1). A major risk factor for atherosclerosis is low-density lipoprotein (LDL) cholesterol. Although there are excellent treatments for elevated LDL cholesterol, therapeutic goals are commonly not met. As targets for lowering of cholesterol become more aggressive, there is a need for more modalities to meet these goals. Lipoprotein (a) [Lp(a)] is an important risk factor, elevated in many patients with premature atherosclerosis, and few therapies lower Lp(a) (2).
Thyroid hormones reduce body weight, LDL cholesterol, and Lp(a); thus, exploitation of these properties may be useful for therapy (3–6). Unfortunately, endogenous thyroid hormones are nonselective and produce undesirable side effects, particularly cardiac stimulation (7, 8). Development of thyromimetics devoid of cardiac effects could have therapeutic potential as antiobesity and lipid-lowering agents.
Thyroid hormone receptors (TRs) are divided into two primary subtypes (TRα and -β), which are the products of two genes of the superfamily of nuclear hormone receptors (4, 7). TRs mediate distinct physiologic effects due to differences in tissue abundance or receptor-specific activity (9). Studies in patients with the syndrome of resistance to thyroid hormone, in which there are abnormal TRβ, and with TRα1–/– mice suggest that TRα is the major TR regulating heart rate (HR) (4, 9–12). TRβ is critical in controlling hepatic cholesterol metabolism and thyroid-stimulating hormone (TSH) suppression, which may be due to high expression of TRβ in liver (70–80% of total TR) and pituitary (10, 12, 13). The TRβ–selective agonist GC-1 reduces plasma cholesterol levels with minimal cardiac effects in mice and rats; however, GC-1 also exhibits relatively decreased uptake into the heart compared with liver, and these differences, rather than TRβ selectivity (14), might account for its selective pharmacology. The effect of GC-1 on metabolic rate has not been reported; therefore, the potential of GC-1 or other TRβ-selective agonists as antiobesity compounds is unclear. Clinically useful TRβ agonists would require high selectivity for cholesterol reduction with modest metabolic rate increases (5–10%) without tachycardia over a wide therapeutic dose range to be safe and efficacious.
The role of TRβ in modulating metabolic rate is currently unclear. GC-1 up-regulates uncoupling protein 1 in brown adipose tissue (15), whereas a study using TRβ–/– mice suggests no effect of TRβ on body weight (16). No studies have measured whole body metabolic rates by using either TRα1–/– mice or TRβ-selective agents.
KB-141 is a TR agonist that binds to the human (h)TRβ with a 14-fold higher affinity than to hTRα (17). In the current studies, we used KB-141 to examine effects of selective activation of TRβ in control and TRα–/– mice, cholesterol-fed rats, and cynomolgus monkeys. The latter more closely resemble humans in terms of lipoprotein metabolism and regulation of body weight (18, 19). 3,5,3′-Triiodi-l-thyronine (T3), the major active form of thyroid hormone, increased the metabolic rate in both WT and TRα–/– mice, but the increase for the WT animals was greater than with the TRα–/– mice. These data imply that both TRα and -β regulate the metabolic rate. As compared with T3, KB-141 reduced plasma cholesterol levels selectively vs. increasing HR in all three models. Relative to T3, KB-141 also increased whole body oxygen consumption (MVO2) in both mice and rats more than HR. In monkeys, KB-141 decreased body weight after 1 wk of treatment by up to 7% and Lp(a) by up to 56% without tachycardia. These studies suggest that selective stimulation of the TRβ might be exploited as a therapeutically effective means to lower weight, plasma cholesterol, and Lp(a) without eliciting deleterious cardiac effects.
Methods
Studies in TRα1–/– Mice. Development of TRα1–/– mice has been described (9–11). After 2 wk of cholesterol feeding (1.5% cholesterol, 0.5% cholic acid), WT or TRα1–/– mice were treated orally with vehicle (10% m-pyrol, 5% ethanol, 5% cremaphor, and 80% water for all studies) or 1.54–462 nmol/kg T3 per day for 7 days (n = 10 per group). Before and after 7 days of treatment, MVO2 was determined in conscious mice by using Oxymax chambers (Columbus Instruments, Columbus, OH) (18, 19). The mice were then anesthetized with i.p. sodium pentobarbital (30 mg/kg), and HRs were measured by using lead II ECG. Afterward, blood samples were removed from the vena cava and analyzed for plasma HDL and LDL cholesterol and blood chemistries (liver enzymes, electrolytes, blood urea nitrogen, etc.), as described (14). TSH could not be measured in mice because no reliable assay existed at the time these studies were performed. An identical study was done by using KB-141 (154–2,920 nmol/kg per day), a TRβ-selective agonist that will be described below.
Previous studies on TRα–/– mice from our laboratories were done in mice instrumented for conscious HR and body temperature measurements with telemetry (10). To reproduce these conditions, animals were implanted with ECG and temperature leads and after recovery, the TRα1–/– and WT mice were treated orally (after 2 wk of cholesterol feeding) with either vehicle or 154 nmol/kg T3 per day for 7 days (n = 5 per group). Body temperatures and HR were monitored throughout the study with telemetry (Datasciences, St. Paul, MN) and at end of the study, MVO2 was determined.
Studies with KB-141. TR-binding affinity studies and reporter cell assay. TR-binding affinities were measured as described (20, 21). Briefly, KB-141 (Fig. 1) was incubated with [125I]T3 (200 pM) and recombinant hTRα1 or -β1 (20 pM) until equilibrium, and unbound ligand was separated from receptor-bound ligand. IC50 values denote the concentration of KB-141 inhibiting 50% of the binding of [125I]T3. The Kd for [125I]T3 is lower for hTRα1 (58 ± 5 pM) than for hTRβ1 (112 ± 8 pM); therefore, the IC50 for an unlabeled compound with equal affinity (Ki) for the two subtypes is lower for hTRβ1 than for hTRα1. The Cheng–Prusoff relationship for a competitive inhibitor was used to obtain the affinity (Ki) for binding to the hTRs (20–22). Normalized hTR selectivity was calculated as IC50 [(hTRα1)/IC50 (hTRβ1) × 1.7].
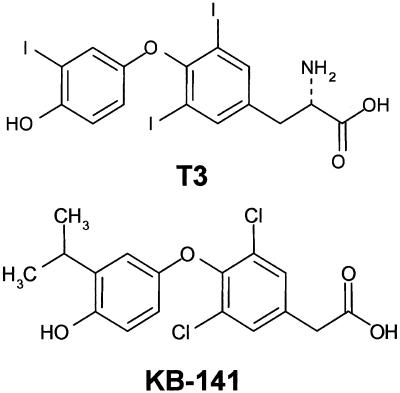
Fig. 1.
Chemical structures of T3 (3,5,3′-triiodo-l-thyronine) and KB-141.
The KB-141 agonist profile was determined by using Chinese hamster ovary K1 cells stably transfected with expression vectors for hTRα1 or -β1 and subsequently with a reporter vector encoding a secreted form of alkaline phosphatase (23, 24) and containing a thyroid hormone response element. The cell lines thyroid hormone alkaline phosphatase (TRAF)-α and -β were exposed to serial dilutions of agonists and incubated for 48 h. Alkaline phosphatase in the cell culture media was then measured by using a chemiluminescent assay (24, 25).
Cholesterol-fed rat studies. Sprague–Dawley rats (250–300 g) were cholesterol-fed for 2 wk and were then dosed once daily with KB-141, T3 (Sigma), or vehicle (n = 5–6 per group) by oral gavage for 7 days. MVO2 was then measured in conscious rats by using Oxymax chambers. Rats were then anesthetized with i.p. sodium pentobarbital (30 mg/kg), and HR was measured by using lead II ECG. Blood was collected and analyzed for cholesterol, TSH, and blood chemistries as described (14). The percent of total cholesterol composed of LDL was >90% after cholesterol feeding.
The relative tissue uptake of T3 and KB-141 was determined. Rats were anesthetized with 30 mg/kg pentobarbital, i.p., and either T3 or KB-141 (10 μmol/kg) was injected into the right jugular vein (n = 3 per group). Plasma and tissue samples were collected 1 h after treatment. Samples were weighed and homogenized with three volumes of deionized water, and tissue concentrations were determined by using liquid chromatography/MS, as described (14).
A separate group of rats was used for isolated heart studies to examine cardiac contractile function and hypertrophy (heart weight/body weight). After 7 days of treatment with vehicle, T3, or KB-141 (1.54–2,920 mol/kg per day, n = 5 per group), hearts were removed and retrogradely perfused with Krebs–Henseleit buffer, and the left ventricles were fitted with a balloon for measurement of developed pressure and HR as described (26).
Studies in Cynomolgus Monkeys. Cynomolgus monkeys were dosed with KB-141 (154, 462, and 924 nmol/kg per day) or T3 (46.2 and 154 nmol/kg per day) (n = 5 per group) orally once daily for 7 days. At the end of this period, animals were anesthetized with ketamine (3–5 mg), and blood pressure and HR were determined by using a pressure cuff at the base of the tail. Blood samples were taken for analysis of cholesterol and blood chemistries.
Results
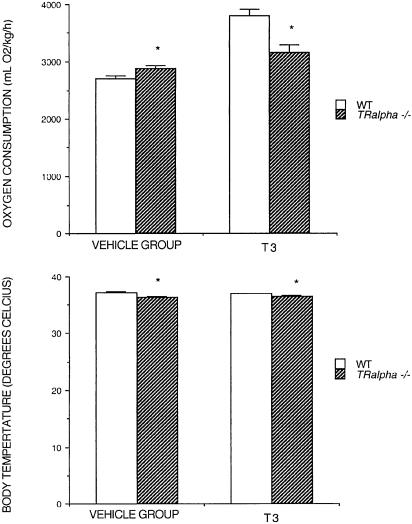
Effect of T3 in Cholesterol-Fed TRα1–/– Mice. We tested the response of conscious TRα1–/– and WT mice (telemetry) to 7 days of 154 nmol/kg T3 per day. Confirming previous results (10, 11), baseline HR was significantly (P < 0.05) lower in TRα1–/– mice, as was body temperature (488 ± 12 vs. 530 ± 4 beats per min and 36.4 ± 0.1 vs. 37.2 ± 0.2°C for TRα–/– and WT, respectively; Fig. 2). Despite a lower body temperature, baseline MVO2 was slightly, but significantly (P < 0.05), higher in the untreated TRα1–/– mice (2,885 ± 51 vs. 2,697 ± 53 ml/kg O2 per hour for vehicle-treated TRα1–/– and WT, respectively; Fig. 2). T3 increased MVO2 and HR to significantly (P < 0.05) higher levels in WT compared with TRα1–/– mice (632 ± 15 and 564 ± 18 beats per min; 3,801 ± 121 and 3,168 ± 118 ml/kg O2 per hour, respectively), although the increase in MVO2 for TRα1–/– mice was significant (P < 0.05). Body temperature at predrug values was maintained in both groups. These observations indicate that T3 acting through TRβ can increase both MVO2 and HR, and that body temperature may not predict changes in MVO2.
Fig. 2.
Oxygen consumption and body temperatures in WT and TRα–/– mice before and after 154 nmol/kg T3 per day (7-day treatment).
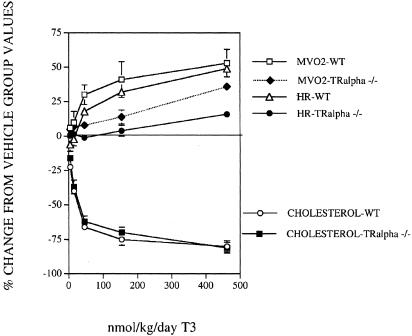
Dose–response studies with T3 in TRα1–/– and WT mice are shown in Fig. 3. T3 had reduced potency on HR in TRα1–/– mice relative to WT (P < 0.05). MVO2 was increased by T3 in a dose-dependent manner in TRα1–/– mice, but the increase was less than in WT mice (P < 0.05); however, the increase in MVO2 was greater than that of HR (Fig. 3). We assigned potency values as ED50 for cholesterol (dose reducing cholesterol 50%), ED15 for HR (dose increasing HR 15%, viewed as the highest acceptable increase), and ED5 for MVO2 (dose increasing MVO2 5%, viewed as lower end of efficacious range). Potency ratios were then calculated for HR vs. MVO2 and vs. cholesterol lowering, showing selectivity (Table 1). MVO2 was increased by 5% with a 4.7-fold selectivity over a 15% increase in HR in WT mice and with a 45.5-fold selectivity vs. a 15% increase in HR in TRα1–/– mice. Thus, the selectivity for a 5% increase in MVO2 vs. HR in TRα1–/– mice normalized for WT mice was 9.7-fold (Table 1). The effects of T3 on cholesterol are similar in TRα1–/– animals relative to WT. This lowering is likely due to a decrease in LDL cholesterol, because >90% of the total plasma cholesterol in these animals is the LDL form (27–32). Thus, under these conditions, cholesterol lowering is primarily regulated by TRβ, MVO2 by both TRβ and -α, and HR mostly by TRα.
Fig. 3.
Effect of T3 on HR, plasma cholesterol, and MVO2 in TRα1–/– mice and WT mice after 7 days of treatment.
Table 1. Potencies and potency ratios for T3 and KB-141 in WT and TRα1-/- mice.
| Compound | Mice | HR ED15 | MVO2 ED5 | Cholesterol ED50 | HR/MVO2 | HR/Cholesterol |
|---|---|---|---|---|---|---|
| T3 | WT | 33.6 | 7.2 | 28.9 | 4.7 | 1.4 |
| TRα1-/- | 469 | 10.3 | 35.8 | 45.5 | 38.2 | |
| KB-141 | WT | 2,838 | 172 | 201 | 16.5 | 14.2 |
| TR α1-/- | 2,556 | 228 | 187 | 11.2 | 13.6 |
All values are mean ± SE and are shown as nmol/kg per day. 45.5/4.7 = 9.5 is selectivity for MVo2 vs. HR normalized for T3 response in WT mice. 38.2/1.2 = 27.2 is selectivity for cholesterol vs. HR normalized for T3 response in WT mice.
Studies with KB-141. Binding affinities and transactivation in vitro. The ability of KB-141 to compete with [125I]T3 for binding to human TRα1 and -β1 is shown in Tables 2, 3, 4. Binding affinity of KB-141 to hTRα1 is 1.7% that of T3 (IC50s for T3 and KB-141 of 0.4 and 23.9, respectively), whereas the binding affinity to hTRβ1 is 27% that of T3 (IC50s for T3 and KB-141 of 0.3 and 1.1, respectively). Thus, the normalized hTRβ1 binding selectivity for KB –141 is 10-fold.
Table 2. Binding affinities calculated from displacement of T3 from full length hTRα and -β.
| Compound | TRα1 IC50, nM | TRβ1 IC50, nM | Normalized TRα/β selectivity |
|---|---|---|---|
| T3 | 0.4 | 0.3 | 0.8 |
| KB-141 | 23.9 | 1.1 | 13.1 |
Table 3. Transactivation in TRAF-α and -β cells.
| Compound | TRAF-α EC50, nM | TRAF-β EC50, nM |
|---|---|---|
| T3 | 1.3 (100% agonism) | 3.5 (100% agonism) |
| KB-141 | 11.2 (101% agonism) | 3.4 (105% agonism) |
Table 4.
Potency ratios for T3 and KB-141 in vivo
| Compound | ED15 HR/ED50 cholesterol | ED15HR/ED5 MVo2 | ED30 TSH/ED50 cholesterol |
|---|---|---|---|
| T3 | 30.8/20.6 = 1.5 | 30.8/21.5 = 1.4 | 6.7/21.6 = 0.3 |
| KB-141 | 2,904/72.3 = 40.3 (27-fold vs. T3) | 2,904/232 = 12.5 (9-fold vs. T3) | 31.8/72.3 = 0.4 (1.3-fold vs. T3) |
All in vivo ED values are shown as nmol/kg per day. ED15 HR is the dose causing 15% increase in HR; ED50 cholesterol is the dose causing 50% reduction in cholesterol; ED5 is the dose causing 5% increase in metabolic rate. ED5 was chosen because it represents the potential therapeutic increase for antiobesity effects. ED30 TSH is the dose causing a 30% reduction in TSH.
KB-141 is a full agonist in both the TRα and -β reporter cell systems (Methods and Table 3). KB-141 is equipotent to T3 in TRAF-β cells (EC50s of 3.5 and 3.4 nM for T3 and KB-141, respectively), but KB-141 has 12% the potency of T3 in TRAF-α cells (EC50s of 1.3 and 11.2 nM for T3 and KB-141, respectively). Thus, the TRβ/-α selectivity KB-141 normalized to that of T3 is 8.3 (Table 3).
Effect of KB-141 in TRα1–/– and WT Mice. KB-141 increased MVO2 with selectivities of 16.5- and 11.2-fold vs. HR in WT and TRα1–/– mice (Table 1). Similar selectivities of 14.2- and 13.6-fold, respectively, were observed for cholesterol lowering vs. HR in both groups of animals (Table 1). KB-141 was less potent for all parameters compared with T3, which is consistent with its lower affinity for TRβ than T3.
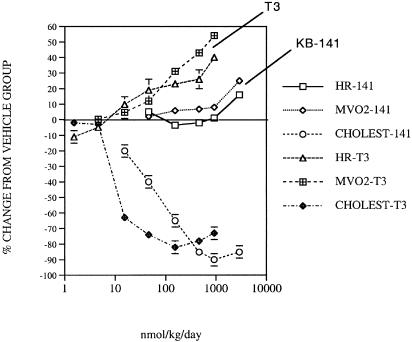
Effect of KB-141 in cholesterol-fed rats. Dose–response effects of T3 and KB-141 on HR, MVO2, and cholesterol lowering in cholesterol-fed rats are shown in Fig. 4 and Table 4. Cholesterol was reduced in a dose-dependent manner by T3 and KB-141 (primarily LDL-cholesterol reduction), with KB-141 being ≈30% as potent as T3 (Table 4), consistent with the lower TRβ-binding affinity for KB-141. HR was increased by T3 and KB-141, although KB-141 was significantly less potent (Table 4). Thus, KB-141 is ≈27-fold more selective for cholesterol lowering vs. tachycardia compared with T3. TSH was reduced by T3 and KB-141 in parallel with cholesterol reduction, consistent with TRβ-dependent actions (Table 4).
Fig. 4.
Effect of T3 or KB-141 (KB) on HR, plasma cholesterol, and MVO2 consumption in cholesterol-fed rats after 7 days of treatment.
MVO2 was increased in a dose-dependent manner by T3 and KB-141 (Fig. 4, Table 4), although the slope of the curve was significantly (P < 0.05) shallower for KB-141 based on the log curve fit. KB-141 has a 9-fold greater selectivity for MVO2 changes compared with HR (potency ratio for ED15 HR/ED5 MVO2, Table 4).
Levels of T3 and KB-141 were determined in samples from a separate group of rats. The tissue/plasma ratios for heart were 1.0 and 0.7; for liver, 7.5 and 5.6; for adipose tissue, 0.2 and 0.3; and for skeletal muscle, 0.6 and 0.4 for T3 and KB-141, respectively. T3 and KB-141 appear to be similar in their abilities to penetrate these tissues, suggesting that the selectivity for cholesterol lowering (and possibly MVO2) is due to TRβ selectivity.
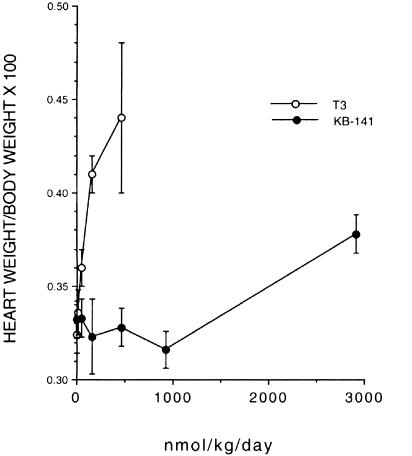
Isolated Rat Heart Studies. Cholesterol-fed rats were treated with T3 or KB-141 for 7 days, after which the hearts were removed and cardiac function ex vivo was determined. There were no differences in body weights among groups. HR changes in isolated perfused hearts mirrored drug effects in vivo such that KB-141 had a lower potency for producing tachycardia (ED15 = 31 vs. 2,893 nmol/kg per day for T3 and KB-141, respectively). KB-141 had no effect on inotropy (contractility) or lusitropy (rate of relaxation) nor did it cause cardiac hypertrophy, except at very high doses (shown as ratio of heart weight to body weight in Fig. 5). By contrast, T3 caused a dose-dependent increase in heart weight/body weight ratio (ED15 = 49 nmol/kg per day), suggesting increased cardiac work. T3 caused a significant and dose-dependent increase in inotropy (e.g., left ventricular developed pressure = 121 ± 4 mm Hg for vehicle and 153 ± 4 mm Hg at 462 nmol/kg T3 per day). These results are consistent with the large increase in MVO2 and direct cardiac effects of T3. Because these effects are not observed with KB-141, it is likely that the T3 effects on heart weight and inotropy are mediated through TRα.
Fig. 5.
Effect of KB-141 or T3 on heart weight/body weight ratio in rats treated for 7 days.
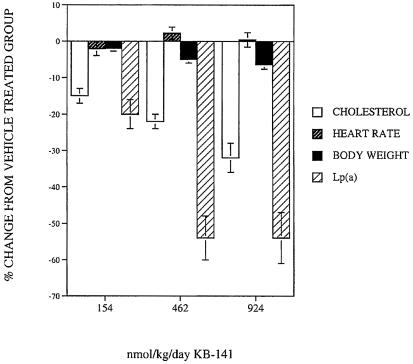
Primate Studies. KB-141 was administered for 7 days to cynomolgus monkeys to determine effects in a model more closely resembling humans. Plasma cholesterol (Fig. 6) was reduced in a dose-dependent manner by KB-141 with a reduction of ≈35% at the highest dose, and most of this reduction was due to reduction in LDL cholesterol. Baseline LDL cholesterol was 49 ± 2 mg/dl, and HDL cholesterol was 43 ± 2 mg/dl. No tachycardia was observed. Despite the short duration of treatment, body weight was also significantly reduced by KB-141 by 5% at the 462 nmol/kg per day dose and 7% at the 924 nmol/kg per day dose compared with paired predrug weights (Fig. 5). The animals were fed ad libitum; food consumption and blood chemistries were normal (blood urea nitrogen, creatinine, electrolytes, liver enzymes, creatine kinase, etc.), and the animals quickly regained their weight after withdrawal of treatment, suggesting that the animals were healthy. No effect of KB-141 on blood pressure was noted. T3 at 46.2 and 154 nmol/kg per day produced cholesterol lowering equivalent to 924 nmol/kg per day KB-141 but with significant tachycardia. KB-141 significantly reduced Lp(a) by ≈50% at the 462- and 924-nmol/kg per day doses (Fig. 6), as did T3 (data not shown).
Fig. 6.
Effect of KB-141 on HR, cholesterol body weight, and Lp(a) in cynomolgus monkeys after 7 days of treatment (46.2, 154 nmol/kg T3 per day = 25%, 24% incr. HR, respectively).
Discussion
We addressed the potential for selectively stimulating TRβ vs. -α for inducing weight loss and lowering plasma cholesterol and Lp(a) levels without excessively stimulating HR, by comparing T3 with the TRβ-selective KB-141. We confirmed that KB-141 was TRβ-selective in both TR-binding and cell-based assays. We used three in vivo model systems: WT and TRα–/– mice, cholesterol-fed rats, and cynomolgus monkeys. Each system offers distinct advantages, and results with the three systems were complementary. For mice and rats, we measured MVO2 as an index of metabolic rate, which contributes to thyroid hormone-induced weight loss (4), and in primates we measured weight.
T3 lowered cholesterol to a similar degree in WT and TRα1–/– mice but showed differences in effects on MVO2 and HR. Whereas T3 induced a large increase in HR in WT mice, the increase was much less for the TRα1–/– mice. T3 increased significantly MVO2 in both the WT and TRα1–/– mice, although the maximal stimulation in the TRα1–/– mice was ≈60% that observed in the WT mice. These data confirm previous results that TRβ primarily regulates plasma cholesterol levels and TRα primarily regulates HR (9–11,14). They also suggest that both TRα and -β can regulate MVO2. In support of the notion that TRβ-selective stimulation might increase MVO2 without increasing HR was the observation that in TRα–/– mice, there is a 10-fold window in which MVO2 could be therapeutically increased by T3 without tachycardia, whereas T3 showed no selectivity in WT mice (Fig. 3).
In comparing the WT and TRα1–/– mice, we surprisingly found that body temperature can be dissociated from MVO2. As shown previously (10, 11), TRα1–/– mice display lower body temperatures than WT mice, but these TRα1–/– mice paradoxically have slightly but significantly higher metabolic rates than WT (Fig. 2). Further, whereas T3 treatment increased MVO2 in both WT and TRα1–/– mice, it did not change body temperature. We do not understand why this dissociation occurs; it is conceivable that the set point for body temperature is altered in TRα1–/– mice. As expected, KB-141 showed similar selectivities for cholesterol and MVO2 in both WT and TRα1–/– mice.
Compared with T3, KB-141 exhibited selective stimulation of MVO2 and reduction of plasma cholesterol levels relative to HR in cholesterol-fed rats. KB-141 was 27-fold more selective for cholesterol lowering (ED50) vs. tachycardia than T3 and 9-fold more selective for the MVO2 response. The greater selectivity of KB-141 for cholesterol lowering vs. effects on MVO2 suggests that both TRα and -β mediate the latter effect.
We also examined effects of T3 and KB-141 on hearts isolated from control and treated animals. Rodent ventricles contain TRβ receptors (33, 34) and therefore might respond to a TRβ agonist. However, KB-141 had no effect on contractility or rate of relaxation. Whereas T3 induced cardiac hypertrophy, this was not observed for KB-141. Therefore modest degrees of metabolic rate increases can be seen without cardiac side effects.
TSH was suppressed by KB-141 in rats and this mirrored cholesterol-lowering. These observations are consistent with TRβ-selective activation being critical for both effects (4). They also imply that in a therapeutic setting, use of a compound like KB-141 would suppress plasma and tissue levels of T3 and T4. Whereas the administered ligand in this example would compensate for the endogenous hormones in terms of TRβ stimulation, it may not appreciably activate TRα, which could therefore cause a relative TRα hypothyroidism; future work may address this.
Actions of one compound previously reported to cause selective TR modulation might be explained in part by selective tissue uptake. GC-1 has selective effects on cholesterol vs. HR in rodents but is not as readily taken up by the rat heart relative to the liver as compared with T3 (14). The thyromimetic, CGS-23425, reduces cholesterol with no thermogenic or HR effects, although the mechanism for this separation is unclear (23). The selectivity for cholesterol lowering vs. tachycardia for SKF-94901 can be partially explained by selective tissue uptake (14, 35). The distribution of KB-141 relative to plasma was equivalent to T3 in heart, liver, muscle, and adipose tissue. Therefore the selectivity for lowering of cholesterol and MVO2 is likely due to the TRβ selectivity of KB-141.
KB-141 was also examined in cynomolgus monkeys that have a lipoprotein profile resembling humans (primarily LDL cholesterol) more closely than rodents. Plasma cholesterol was reduced in a dose-dependent manner by KB-141 with a reduction of ≈35% at the highest dose. No tachycardia was observed, showing selectivity for cholesterol lowering for KB-141, unlike T3. In the monkeys, KB-141 also lowered Lp(a) levels by 50%. Lp(a) has been suggested previously as a target for nonselective thyroid agonists (6). Thus these atherogenic particles may be regulated by TRβ, and this class of thyromimetics may deserve further exploration as a regulator of Lp(a), for which current therapies have limited efficacy.
Monkeys are also a better model than rodents for examining effects on weight loss, because the animals are not growing. Body weight was significantly reduced by KB-141 by 5% at the 462 nmol/kg per day dose and 7% at the 924 nmol/kg per day dose compared with paired predrug weights, most likely due to increased MVO2. Muscle loss and frank myopathy can be observed in hyperthyroidism (4), but the KB-141-treated animals appeared healthy and maintained their pretreatment level of food consumption, and body chemistries (electrolytes, renal function indices, blood urea nitrogen, and liver functions) remained normal. Thus, it is unlikely that the weight loss was due to a toxic effect of KB-141. Interestingly, little TRβ mRNA is reported in skeletal muscle in humans (4).
In summary, our results in three species show that a separation between potential beneficial vs. deleterious effects of TR stimulation is possible. These effects include reductions of plasma cholesterol and Lp(a) and weight. It is likely in all three cases that the separation is due to the ability of KB-141 to selectively activate TRβ vs. -α. Thus, selective thyromimetics may offer several types of therapeutic potential and are candidates for further exploration.
Acknowledgments
We thank Dr. Kamelia Behnia for help with the pharmacokinetic work and Ms. Patricia Catanzariti for help with the primate Lp(a) assay.
Abbreviations: TR, thyroid hormone receptors; hTR, human TR; HR, heart rate; LDL, lowdensity lipoprotein; TSH, thyroid-stimulating hormone; MVO2, whole body oxygen consumption; Lp(a), lipoprotein (a); T3, 3,5,3′-triiodi-l-thyronine; TRAF, thyroid hormone alkaline phosphatase.
References
- 1.Grundy, S. M. (2000) Endocrine 13, 155–165. [DOI] [PubMed] [Google Scholar]
- 2.Danesh, J., Collins, R. & Peto, R. (2000) Circulation 102, 1082–1085. [DOI] [PubMed] [Google Scholar]
- 3.Davignon, J. (1995) Diabetes Metab. 21, 139–146. [Google Scholar]
- 4.Yen, P. M. (2001) Physiol. Rev. 81, 1097–1142. [DOI] [PubMed] [Google Scholar]
- 5.Engler, H. & Riesen, W. F. (1993) Clin. Chem. 39, 2466–2469. [PubMed] [Google Scholar]
- 6.De Bruin, T. W. A., van Barlingen, van Linde-Sibenius Trip, M., van Vuurst de Vries, A. R., Akveld, M. J. & Erkelens, D. W. (1993) J. Clin. Endocrinol. Metab. 76, 121–126. [DOI] [PubMed] [Google Scholar]
- 7.Lazar, M. A. (1993) Endocr. Rev. 14, 348–399. [DOI] [PubMed] [Google Scholar]
- 8.Klein, I. & Ojamaa, K. (2001) N. Engl. J. Med. 344, 501–509. [DOI] [PubMed] [Google Scholar]
- 9.Forrest, D. & Vennström, B. (2000) Thyroid 10, 41–51. [DOI] [PubMed] [Google Scholar]
- 10.Wikström, L., Johansson, C., Salto, C., Barlow, C., Barros, A., Bass, F., Forrest, D., Thoren, P. & Vennström, B. (1998) EMBO J. 17, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson, C., Vennström, B. & Thoren, P. (1998) Am J. Physiol. 275, R640–R646. [DOI] [PubMed] [Google Scholar]
- 12.Gloss, B., Trost, S., Bluhm, W., Swanson, E., Clark, R., Winkfein, R., Janzen, K., Giles W., Chassande O., Samarut, J. & Dillmann, W. (2001) Endocrinology 142, 544–551. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz, H. L., Strait, K. A., Ling, N. C. & Oppenheimer, J. H. (1992) J. Biol. Chem. 267, 11794–11799. [PubMed] [Google Scholar]
- 14.Trost, S. U., Swanson, E., Gloss, B., Wang-Iverson, D. B., Zhang, H., Volodarsky, T., Grover, G. J., Baxter, J. D., Chiellini, G., Scanlan, T. S., et al. (2000) Endocrinology 141, 3057–3064. [DOI] [PubMed] [Google Scholar]
- 15.Ribeiro, M. O., Carvalho, S. D., Schultz, J. J., Chiellini, G., Scanlan, T. S., Bianco, A. C. & Brent, G. A. (2001) J. Clin. Invest. 108, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss, R. E., Murata, Y., Cua, K., Hayashi, AY., Seo, H. & Refetoff, S. (1998) Endocrinology 139, 4945–4952. [DOI] [PubMed] [Google Scholar]
- 17.Ye, L., Mellstrom, K., Bladh, L., Koehler, K., Garg, N., Collazo, A., Litten, C., Husman, B., Persson, K, Ljunggren, J., et al. (2003) J. Med. Chem. 46, 1580–1588. [DOI] [PubMed] [Google Scholar]
- 18.Iossa, S., Liverini, G. & Barletta, A. (1992) J. Endocrinol. 135, 45–51. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheimer, J. H., Schwartz, H. L., Lane, J. T. & Thompson, M. P. (1991) J. Clin. Invest. 87, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenidge, P. A., Carlsson, B., Bladh, L. G. & Gillner, M. J. (1998) Med. Chem. 14, 2503–2512. [DOI] [PubMed] [Google Scholar]
- 21.Chiellini, G., Apriletti, J. W., Yuoshihara, H. A., Baxter, J. D., Ribeiro, R. C. J. & Scanlan, T. S. (1998) Chem. Biol. 5, 299–306. [DOI] [PubMed] [Google Scholar]
- 22.Barkhem, T. Carlsson, B. Simons, J. Moeller, B. Berkenstam, A. Gustafsson, J.-Å. & Nilsson, S. J. (1991) Steroid Biochem. Mol. Biol. 38, 667–675. [DOI] [PubMed] [Google Scholar]
- 23.Baxter, J. D., Goede, P., Apriletti, F. W., West, B. L., Feng, W., Mellström, K., Fletterick, R. J., Wagner, R. L., Kushner, P. J., Ribeiro, R. C. J., et al. (2002) Endocrinology 143, 517–524. [DOI] [PubMed] [Google Scholar]
- 24.Berger, J., Hauber, J., Hauber, R., Geiger, R. & Cullen, C. (1988) Gene 66, 1–10. [DOI] [PubMed] [Google Scholar]
- 25.Barkhem, T., Carlsson, B., Nilsson, Y., Enmark, E., Gustafsson, J.-Å. & Nilsson, S. (1998) Mol. Pharmacol. 54, 105–112. [DOI] [PubMed] [Google Scholar]
- 26.Grover, G. J., D'Alonzo, A., Garlid, K., Bajgar, R., Lodge, N., Sleph, P., Darbenzio, R., Hess, T., Smith, M., Paucek, P., et al. (2001) J. Pharmacol. Exp. Ther. 297, 1184–1192. [PubMed] [Google Scholar]
- 27.Underwood, A. H., Emmett, J. C., Ellis, D., Flynn, S. B., Leeson, P. D., Benson, G. M., Novelli, R., Pearce, N. J. & Shah, V. P. (1986) Nature 324, 425–429. [DOI] [PubMed] [Google Scholar]
- 28.Stephan, Z. F., Yurachek, E. C., Sharif, R., Wasvary, J. M., Leonards, K. S., Hu, C, Hintze, T. H. & Steele, R. E. (1996) Atherosclerosis 126, 53–63. [DOI] [PubMed] [Google Scholar]
- 29.Steele, R. E. (1995) in Atherosclerosis X, eds. Woodford, F. P., Davignon, J. & Sniderman, A. (Elsevier Science, Amsterdam), pp. 321–324.
- 30.Rakow, A. D., Klor, H. U., Kuter, E., Ditschuneit, H. H. & Kitscheneit, H. (1976) Atherosclerosis 24, 369–380. [DOI] [PubMed] [Google Scholar]
- 31.Scarabottolo, L., Trezzi, E., Roma, P. & Catapan, A. L. (1986) Atherosclerosis 59, 329–333. [DOI] [PubMed] [Google Scholar]
- 32.Ridgway, N. D. & Dolphin, P. J. (1985) J. Lipid Res. 26, 1300–1313. [PubMed] [Google Scholar]
- 33.Erem, C., Deger, O., Bosgtan, M., Orem, A., Sonmez, M., Ulusoy, S. & Telatar, M. (1999) Acta Cardiol. 54, 77–81. [PubMed] [Google Scholar]
- 34.Shahrara, S. & Drvota, V. (1999) J. Cardiovasc. Pharmacol. 34, 261–267. [DOI] [PubMed] [Google Scholar]
- 35.Barlow, J. W., Raggatt, L. E., Lim, C.-F., Kolliniatis, E., Topliss, D. J. & Stockigt, J. R. (1991) Acta Endocrinol. 124, 37–44. [DOI] [PubMed] [Google Scholar]